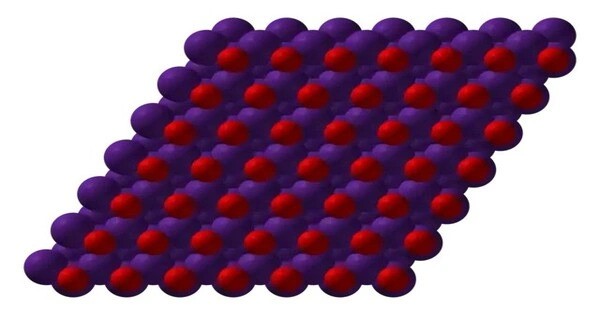
Caesium monoxide or caesium oxide is a chemical compound with the chemical formula Cs2O. It is a highly reactive and unstable substance, typically found as a solid under normal conditions. It is the simplest and most common oxide of the caesium. It forms yellow-orange hexagonal crystals. Caesium, being an alkali metal, has a strong tendency to react with oxygen, and caesium monoxide is one of the compounds that can form when cesium is exposed to oxygen.
Because of its reactivity, caesium monoxide is not commonly encountered outside of specialized laboratory environments. It can be formed by heating cesium metal in an oxygen atmosphere or by reacting cesium with oxygen-containing compounds.
Properties
Caesium monoxide is typically a white to yellow solid, although its exact appearance can vary depending on the preparation and conditions. It’s quite reactive, especially with water, and it forms caesium hydroxide (CsOH) and hydrogen gas when it comes into contact with moisture. As an alkali metal oxide, caesium monoxide is highly basic, reacting readily with water to form a strong base, caesium hydroxide.
- Chemical formula: Cs2O
- Molar mass: 281.810 g·mol−1
- Appearance: Yellow-orange solid
- Density: 4.65 g/cm3, solid
- Melting point: 490 °C (914 °F; 763 K) (under N2)
- Solubility in water: Reacts to form CsOH
Reactions
Elemental magnesium reduces caesium oxide to elemental caesium, forming magnesium oxide as a side-product:
Cs2O + Mg → 2 Cs + MgO
Cs2O is hygroscopic, forming the corrosive CsOH on contact with water.
Occurrences
Caesium monoxide is not commonly found in nature because it’s a relatively unstable compound due to caesium’s high reactivity. It is typically synthesized in the laboratory through the reaction of caesium metal with oxygen. Caesium is a rare element in the Earth’s crust, so compounds like caesium monoxide are not abundant in natural mineral forms.
Uses
Caesium oxide is used in photocathodes to detect infrared signals in devices such as image intensifiers, vacuum photodiodes, photomultipliers, and TV camera tubes L. R. Koller described the first modern photoemissive surface in 1929–1930 as a layer of caesium on a layer of caesium oxide on a layer of silver. It is a good electron emitter; however, its high vapor pressure limits its usefulness.