Caesium auride is the inorganic compound with the formula CsAu. It is the Cs+ salt of the unusual Au− anion. It is a chemical compound formed by combining caesium (Cs), an alkali metal, and gold (Au), a noble metal. It’s a notable compound due to its relatively rare occurrence and interesting properties.
Properties
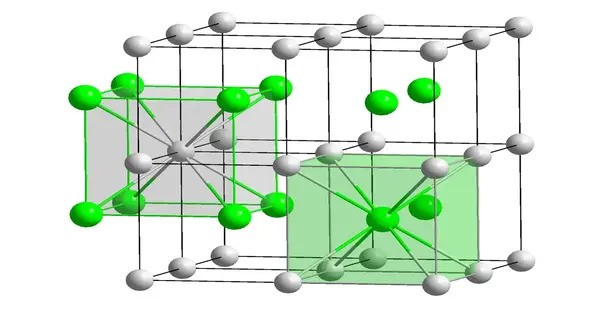
Caesium auride is typically described as a salt-like compound, with a structure similar to that of cesium halides. It crystallizes in a cubic lattice structure. It appears as a yellowish solid, which is a characteristic of gold-containing compounds. It is highly reactive, particularly with water. It reacts with moisture to release hydrogen gas and form caesium hydroxide and gold. It is not very soluble in water due to its ionic nature, but it can be soluble in organic solvents.
- Chemical formula: AuCs
- Molar mass: 329.872022 g·mol−1
- Appearance: Yellow crystals
- Melting point: 580 °C (1,076 °F; 853 K)
- Solubility in water: reacts violently
Preparation and reactions
CsAu is obtained by heating a stoichiometric mixture of caesium and gold. The two metallic-yellow liquids react to give a transparent yellow product. Despite being a compound of two metals, CsAu lacks metallic properties since it is a salt with localized charges; it instead behaves as a semiconductor with band gap 2.6 eV.
The compound hydrolyzes readily, yielding caesium hydroxide, metallic gold, and hydrogen.
2 CsAu + 2 H2O → 2 CsOH + 2 Au + H2
The solution in liquid ammonia is brown, and the ammonia adduct CsAu·NH3 is blue; the latter has ammonia molecules intercalated between layers of the CsAu crystal parallel to the (110) plane. Solutions undergo metathesis with tetramethylammonium loaded ion exchange resin to give tetramethylammonium auride.
Formation and Synthesis
- Laboratory Synthesis: Caesium auride is typically prepared by reacting caesium metal with gold. The process involves heating caesium and gold together, leading to the formation of the auride.
- Alternative Methods: It can also be synthesized by reacting caesium hydroxide with gold chloride in a controlled environment.
Occurrences
- Natural Occurrence: Caesium auride is extremely rare in nature. It is not typically found in large quantities and is not known to occur in significant natural deposits.
- Synthetic Occurrence: The compound is mainly synthesized in laboratories for research purposes or in experimental setups where caesium and gold are used in various chemical investigations.
Applications
- Research and Chemistry: Caesium auride, like other aurides, is mainly used in scientific research, particularly in the study of metallic bonding and ionic compounds.
- Electronics: Due to its conductivity properties when molten, caesium auride can also be of interest in electronic and material science applications, although its rarity limits practical use.
Safety and Handling
- Reactivity: Being an alkali metal auride, caesium auride is very reactive, especially with water and moisture. It can release caustic caesium hydroxide and hydrogen gas when it reacts with water.
- Precautions: Proper handling precautions are necessary, including working in a controlled environment, wearing protective gear, and ensuring that it does not come into contact with moisture.