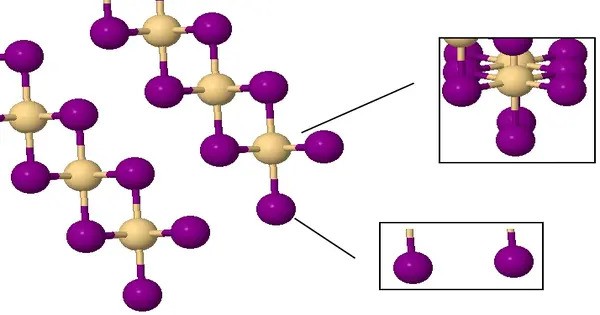
Cadmium iodide is an inorganic compound with the formula CdI2. It is usually a yellowish-white crystalline solid. It is a white hygroscopic solid. It also can be obtained as a mono- and tetrahydrate. It has few applications. It is notable for its crystal structure, which is typical for compounds of the form MX2 with strong polarization effects. It is hygroscopic, meaning it can absorb moisture from the air.
The compound exhibits semiconducting properties, which are of interest in electronic applications. It’s used in the manufacture of thin-film solar cells. It can be used in some semiconductor applications. It serves as a reagent in chemical synthesis and various laboratory applications.
Properties
- Chemical formula: CdI2
- Molar mass: 366.22 g/mol
- Appearance: white to pale yellow crystals
- Density: 5.640 g/cm3, solid
- Melting point: 387 °C (729 °F; 660 K)
- Boiling point: 742 °C (1,368 °F; 1,015 K)
- Solubility in water: 787 g/L (0 °C), 1250 g/L (100 °C)
- Solubility: soluble in ethanol, acetone, ether and ammonia
Preparation
Cadmium iodide is prepared by the addition of cadmium metal, or its oxide, hydroxide or carbonate to hydroiodic acid. Also, the compound can be made by heating cadmium with iodine. CdI₂ can be produced by reacting cadmium salts (like cadmium carbonate or cadmium oxide) with hydroiodic acid.
Occurrences
Cadmium is not found in nature in its elemental form; it primarily occurs as a byproduct of zinc, lead, and copper extraction. Therefore, cadmium iodide is not typically found in nature but can be synthesized.
Applications
Historically, cadmium iodide was used as a catalyst for the Henkel process, a high-temperature isomerisation of dipotassium phthalate to yield the terephthalate. The salt was then treated with acetic acid to yield potassium acetate and commercially valuable terephthalic acid.
While uneconomical compared to the production of terephthalic acid from p-xylene, the Henkel method has been proposed as a potential route to produce terephthalic acid from furfural. As existing Bio-PET is still reliant on petroleum as a source of p-xylene, the Henkel process could theoretically offer a completely bioplastic route to polyethylene terephthalate.
Safety
Cadmium compounds are toxic and can pose health risks if inhaled or ingested. Proper safety precautions should be taken when handling it.