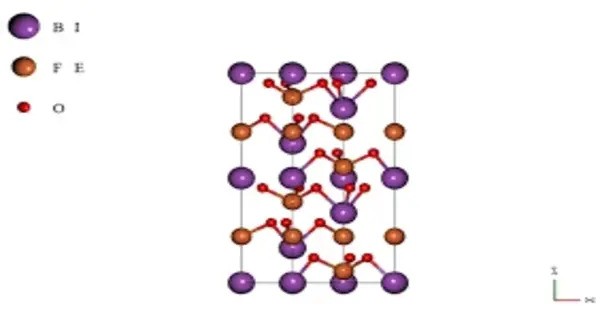
Bismuth ferrite (BiFeO3, also commonly referred to as BFO in materials science) is an inorganic chemical compound with perovskite structure and one of the most promising multiferroic materials. It’s a type of multiferroic material, meaning it exhibits both ferroelectric and magnetic properties. The room-temperature phase of BiFeO3 is classed as rhombohedral belonging to the space group R3c. This dual functionality makes it useful for various applications, including in memory devices, sensors, and actuators.
It is synthesized in bulk and thin film form and both its antiferromagnetic (G type ordering) Néel temperature (approximately 653 K) and ferroelectric Curie temperature are well above room temperature (approximately 1100K). Ferroelectric polarization occurs along the pseudocubic direction (⟨111⟩c with a magnitude of 90–95 μC/cm2.
Properties
- Chemical formula: BiFeO3
- Molar mass: 312.822 g·mol−1
- Multiferroic Behavior: It has both ferroelectric (electric polarization that can be reversed by an electric field) and antiferromagnetic (magnetic ordering in which adjacent magnetic moments point in opposite directions) properties.
- High Curie Temperature: Bismuth ferrite has a high Curie temperature (the temperature above which it loses its ferroelectric properties), around 830°C, which makes it stable at elevated temperatures.
- Large Polarization: It exhibits a large spontaneous polarization, which is beneficial for applications that require strong electric fields.
Bismuth Ferrite does not commonly occur in nature. It is generally synthesized in the laboratory for research and industrial purposes. It can be synthesized using various methods such as solid-state reactions, sol-gel techniques, and hydrothermal methods.
Applications
- Due to its multiferroic nature, Bismuth Ferrite is studied for applications in magnetic sensors, memory devices, and spintronic devices.
- Its ferroelectric properties make it useful in non-volatile memory and piezoelectric applications.