The superhard material beta carbon nitride (β-C3N4) is predicted to be harder than diamond. It is a hypothetical binary carbon-nitrogen compound, CN, that is thought to have properties similar to diamond or boron nitride.
Amy Liu and Marvin Cohen proposed the material for the first time in 1985. They hypothesized that carbon and nitrogen atoms could form a particularly short and strong bond in a stable crystal lattice in a 1:1.3 ratio by studying the nature of crystalline bonds. It was proposed in 1989 that this material would be harder than diamond on the Mohs scale.
For many years, the material was thought to be difficult to produce and could not be synthesized. The production of beta carbon nitride was recently accomplished. For example, mechanochemical processing was used to create nanosized beta carbon nitride crystals and nanorods of this material.
Characteristics
Structure
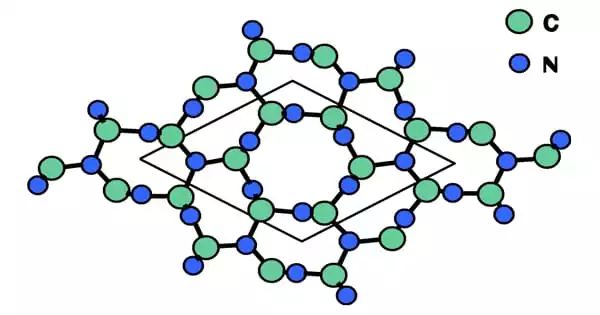
Fourier transformation infrared spectroscopy, transmission electron microscopy, and X-ray diffraction were used to determine the structure. A polycrystalline β-C3N4 with a lattice constant of a = 6.36 Å, c = 4.648 Å can be determined using a SAED. Thermal annealing can transform flake-like structures into sphere- or rod-like structures.
It has the same crystal structure as β-Si3N4 and consists of a hexagonal network of tetrahedrally (sp3) bonded carbon and trigonal planar nitrogen (sp2).
The nanorods are generally straight and free of flaws.
Properties
A hardness comparable to or greater than that of diamond (the hardest known material) has been predicted but has yet to be demonstrated.
Diamond has a bulk modulus of 4.43 MBar, whereas β-C3N4 has a bulk modulus of 4.27 MBar (±.15). This is the closest bulk modulus to diamond that has been conceived.
Possible applications
- Exciting prospects in tribology, wear resistant coating, optical engineering, and electronic engineering.
- There are also composite opportunities using TiN as seeding layers for carbon nitride, which results in actual crystalline composites with hardness levels of 45-55 (GPa), which is on the lower end of diamond.
- The predicted hardness of pure beta carbon nitride (4.27 ± .15 Mbar) is comparable to that of diamond (4.43 Mbar), indicating that it has the potential to be useful in the same fields as diamond.
- It can be used in tribological coatings, biocompatible medical coatings, chemically inert coatings, insulators, and energy-storage solutions in its micron-sized graphitic form. One of the best hydrogen storage materials is graphitic carbon nitride.