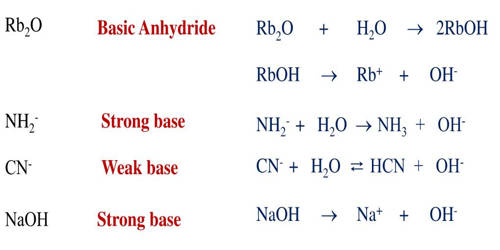
A base anhydride is an oxide of a chemical element from group 1 or 2 (the alkali metals and alkaline earth metals, respectively). It is a metal oxide that forms a basic solution when reacted with water. They are obtained by removing water from the corresponding hydroxide base. If water is added to a base anhydride, a corresponding hydroxide salt can be re-formed. Examples: An example of a base anhydride is CaO, which turns into CaOH in water. The oxides to demonstrate periodicity with respect to acidity. In solution, they form molecular acids.
Base anhydrides are not Brønsted–Lowry bases because they are not proton acceptors. They are usually oxides of metals, that can react with water to form bases, or behave toward acids as if they had. However, they are Lewis bases, because they will share an electron pair with some Lewis acids, most notably acidic oxides. They are potent alkalis and will produce alkali burns on the skin because their affinity for water (that is, their affinity for being slaked) makes them react with body water. If the metal is an alkali or alkaline earth, the reaction probably happens quickly and produces a lot of heat. If the metal is a transition metal, the reaction might not happen so easily or at all.

For example, quicklime (calcium oxide) reacts with the skin to become hydrated lime (calcium hydroxide), which is a strong base, chemically akin to lye. Sulfur and nitrogen oxides are considered air pollutants as they react with atmospheric water vapor to produce. Once in the air, sulfur dioxide reacts with oxygen to form sulfur trioxide. This is the acid anhydride in our reaction.
Explanation: The best-known example is calcium oxide CaO which will react with water to form calcium hydroxide Ca(OH)2.
Other metal oxides will not react directly with water, but they will react with acid as if they were a base:
ZnO+2H+→Zn2++H2O (compare to- Zn(OH)2+2H+→Zn2++2H2O)
In this case, you can ‘deduce’ the imaginary in-between step from oxide to base. Some non-metal oxides, such as (N2O) and (CO), do not display any acid/base characteristics. It is important to realize that not all non-metal oxides are acid anhydrides, only those that can react with water. For example, carbon monoxide is an oxide of carbon but does not react with water. Therefore, it is not an acid anhydride.