Barium orthotitanate is an inorganic substance having the chemical formula Ba2TiO4. It is a colorless solid that is of relevance due to its link to barium titanate, a valuable electroceramic. It is a white crystalline substance that is utilized in hearing aids, phonograph pickups, and ceramic transducers.
It is an essential functional material in the electronics sector because to its exceptional dielectric, ferroelectric, piezoelectric, pyroelectric, and electro-optical properties.
Properties
Room temperature entropy – 47.0 cal/deg. mol
Dielectric constant – 20 (at 100 kHz)
It is hygroscopic and decomposes with swelling in moist air.
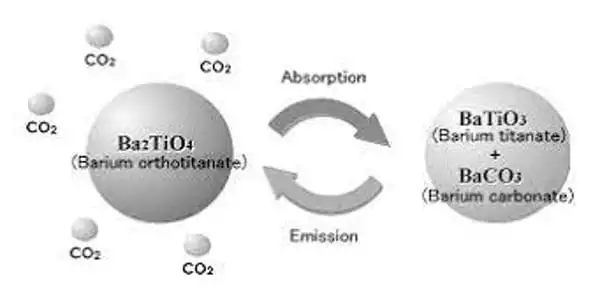
Barium orthotitanate can remove up to 99.9% of CO2 from a high-temperature gas stream by the reaction:
Ba2TiO4 + CO2 → BaTiO3 + BaCO3
It undergoes a phase shift from tetrahedral to cubic at the Curie temperature. Single crystals of barium titanate have also been reported to have negative temperature co-efficients of resistivity (NTCR). It also has ferroelectric characteristics and works well as a photorefractive material.
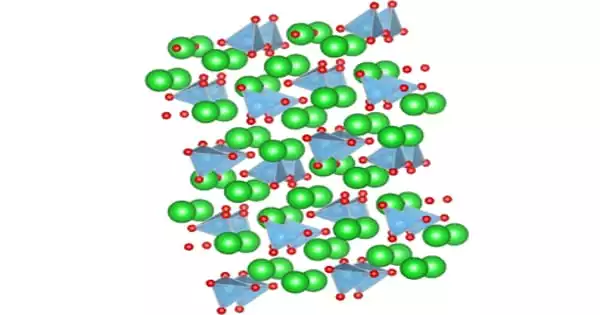
Structure
There are two known phases of the solid: a low-temperature (β) phase with P21/n symmetry and a high-temperature (α′) phase with P21nb symmetry. Ba2TiO4 has an uncommon structure among titanates because its titanium atoms are arranged in a four-oxygen tetrahedron rather than a six-oxygen octahedron.
Depending on the temperature, the solid occurs in one of four polymorphs. From high to low temperatures, the four polymorphs’ crystal symmetries are cubic, tetragonal, orthorhombic, and rhombohedral. Except for the cubic phase, all of these phases exhibit the ferroelectric effect.
Production
It crystallizes as white crystals from a melt of BaCl2, BaCO3, and TiO2 or by sintering BaCO3 and TiO2. Another technique of preparation is to heat Ba(OH)2 with TiO2 pellets. There are also polymer precursor, sol-gel, and reverse micellar approaches for Ba2TiO4 production. Chemical vapor deposition has also been used to successfully create Ba2TiO4 thin films.
It dissolves in a wide range of acids, including sulfuric, hydrochloric, and hydrofluoric acids. It is insoluble in acids and bases, as well as in water. It is an electrical insulator in its pure state. However, it becomes semiconducting when doped with minute amounts of metals, most notably scandium, yttrium, neodymium, samarium, and others.
Application
When formed as big crystals, barium titanate appears white as a powder and is transparent. It’s a ferroelectric ceramic material with photorefractive and piezoelectric characteristics. Capacitors, electromechanical transducers, and nonlinear optics all make use of it.