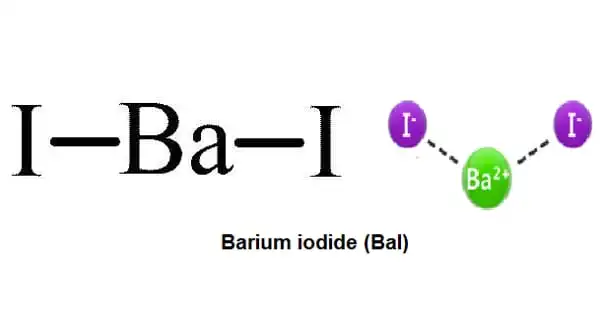
Barium iodide has the formula BaI2 and is an inorganic chemical. It is produced by the breakdown of barium with metallic sodium. The chemical exists in two forms: anhydrous [BaI2(H2O)2] and hydrate [BaI2(H2O)2]. Both are white solids. When heated, hydrated barium iodide turns to anhydrous barium iodide. The hydrated form of barium iodide is widely known to be soluble in water, ethanol, and acetone. The hydrated form is water, ethanol, and acetone soluble.
Barium iodide is commonly employed in the production of other essential iodine compounds. This chemical is also utilized in the production of barium dioxide.
Properties
According to the formula for barium iodide, the molecular weight of the anhydrous form is 391.136 g/mol. Similarly, the hydrated form’s molar mass, as determined from the barium iodide formula, is 427.167 g/mol.
- Density: 5.15 g/cm³
- Molecular Weight/ Molar Mass: 391.136 g/mol
- Boiling Point: 2,027 °C
- Melting Point: 711 °C
- Odour: Odourless
- Appearance: White solid
- pH: 7
- Solubility: Readily soluble in alcohol
Structure
Barium iodide crystallizes as small, colorless needles that deliquesce gently and are extremely soluble in water. The anhydrous form has a crystalline packing structure similar to that of lead(II) chloride, with each Ba core coupled to nine iodide ligands.

Preparation
We can dehydrate barium iodide by combining ammonium carbonate and barium hydroxide in the presence of a hydrogen periodate reagent. We can make anhydrous barium iodide by reacting Ba metal with 1, 2-diiodoethane in ether. It interacts with alkyl potassium compounds to generate organobarium compounds. Furthermore, we can reduce it using lithium biphenyl to produce a highly active version of barium metal.
Reactions
Anhydrous BaI2 can be prepared by treating Ba metal with 1,2-diiodoethane in ether.
BaI2 reacts with alkyl potassium compounds to form organobarium compounds.
BaI2 can be reduced with lithium biphenyl, to give a highly active form of barium metal.
Barium iodide reacts with potassium bromide forming yellow precipitate of potassium iodide and barium bromide.
KBr + BaI2 → KI + BaBr2
Barium sulfate is formed when barium iodide is treated with sodium sulfate. The chemical equation is given below.
BaI2 + Na2SO4 → BaSO4 + NaI
Uses
Barium iodide is toxic in nature just like other soluble salts containing barium. It has very limited uses as compared to many other halide forms.
- Used in the preparation of barium dioxide, but has no medicinal use
- Used in the manufacture of other iodide compounds.
- Used to densify the copper castings described in a paper before the Electrochemical Society.
- It can also be used for the identification of copper castings as has been pointed out by some researchers.
Safety
Barium iodide, like other soluble salts of barium, is poisonous. It can be harmful to one’s health in both hydrous and anhydrous forms. As a result, specific measures must be taken when handling them. If it comes into direct contact with the eyes or skin, it causes irritation. Inhaling or ingesting cerium iodide can result in major health consequences.