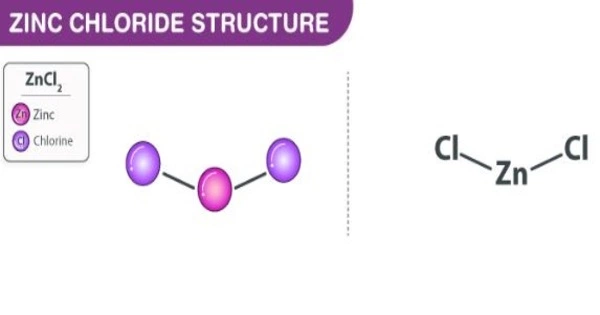
The name zinc chloride refers to chemical compounds with the formula ZnCl2 and their hydrates. It has hygroscopic properties, which means it attracts and captures water molecules from its surroundings. As a result, protecting zinc chloride from moisture is critical.
Zinc chlorides, which exist in nine crystalline forms, are colorless or white and highly soluble in water. This white salt has a high hygroscopicity and is even deliquescent. As a result, samples should be shielded from moisture sources such as water vapor in the air. It has a wide range of applications, including textile processing, metallurgical fluxes, and chemical synthesis.
Properties
Zinc chloride is solid at room temperature and has a white crystalline appearance. It is odourless. The solubility of this compound in water corresponds to 432g/100g. It is also soluble in acetone, ethanol, and glycerol. Molten zinc chloride is highly viscous and has a relatively low electrical conductivity value.
- Molar Mass: 136.315 grams per mole
- Density: 2.907 grams per cubic centimetre
- Melting Point: 563 K (290oC)
- Boiling Point: 1005 K (732oC)

Preparation and purification
Zinc and hydrogen chloride can be used to produce anhydrous ZnCl2. Anhydrous zinc chloride is produced by the reaction of metallic zinc and hydrogen chloride gas. This reaction’s chemical equation is:
Zn + 2 HCl → ZnCl2 + H2
Hydrochloric acid can be used to easily prepare hydrated forms and aqueous solutions of Zn metal, zinc carbonate, zinc oxide, and zinc sulfide. Instead of hydrogen chloride, hydrochloric acid can be used to treat zinc in order to obtain a hydrated form of the compound. When hydrogen chloride reacts with zinc sulphide, zinc chloride and hydrogen sulphide are formed. This reaction’s chemical equation is:
ZnS + 2 HCl + 4 H2O → ZnCl2(H2O)4 + H2S
Unlike many other elements, zinc essentially exists in only one oxidation state, 2+, making chloride purification easier.
Impurities in commercial zinc chloride samples typically include water and hydrolysis products. Recrystallization from hot dioxane can be used to purify such samples. Sublimation in a stream of hydrogen chloride gas followed by heating the sublimate to 400 °C in a stream of dry nitrogen gas can be used to purify anhydrous samples. Finally, the most basic method involves treating zinc chloride with thionyl chloride.
Uses
Zinc chloride is used as a flux/cleaning agent for soldering because it reacts with metal oxides to produce compounds with the general formula MZnOCl2 (where M is the metal). These fluxes have the ability to dissolve the oxide layer on the metal’s surface. Other applications for this compound are listed below –
- This compound acts as a catalyst for some aromatization reactions when molten. Hexamethyl benzene, for example, can be produced from methanol using molten ZnCl2.
- Because it is a moderately strong Lewis acid, it can act as a catalyst for the Fischer indole synthesis reaction and some Friedel-Crafts acylation reactions.
- A solution of anhydrous zinc chloride and concentrated hydrochloric acid is used to make the Lucas reagent. This reagent is extremely helpful in the synthesis of alkyl chlorides.
- Some smoke grenades contain a mixture of zinc oxide and hexachloroethane. When these compounds are ignited, they react to produce zinc chloride smoke, which serves as a smokescreen.
- ZnCl2 is also useful in fingerprint detection since it forms an easily detectable complex with Ruhemann’s purple.