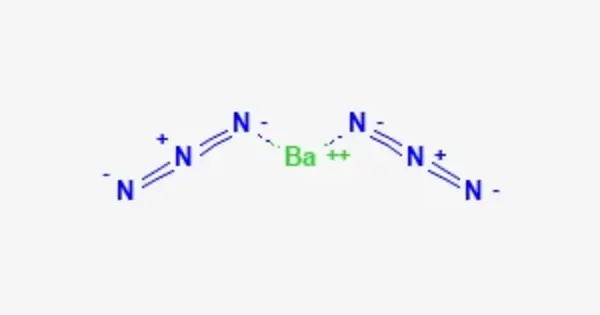
Barium azide is an inorganic azide with the formula Ba(N3)2. It is a barium salt of hydrazoic acid. Like all azides, it is explosive. It is less sensitive to mechanical shock than lead azide. It’s typically a white, crystalline solid and is highly toxic and sensitive, especially when it comes into contact with heat or shock, which can cause it to decompose explosively. Because of these properties, it is primarily used in research and very specialized applications, such as in some pyrotechnic formulations or for initiating explosive reactions.
Preparation
Barium azide may be prepared by reacting sodium azide with a soluble barium salt:
BaBr2 + 2 NaN3 → Ba(N3)2 + 2NaBr
Properties
Barium Azide is usually a white or colorless crystalline solid. It is soluble in water. It is relatively stable but is highly sensitive to shock, friction, and heat. Barium Azide can decompose explosively when subjected to external stimuli, especially in larger quantities. This makes handling it with care important.
- Chemical formula: Ba(N3)2
- Molar mass: 221.37 g/mol
- Appearance: White crystalline solid
- Odor: Odourless
- Density: 2.936 g/cm3
- Melting point: 126 °C (259 °F; 399 K)
- Boiling point: 160 °C (320 °F; 433 K) (initial decomposition) >217 °C (deflagrates), 180 °C (initial decomposition), 225 °C explosion
- Solubility in water: 11.5 g/100 mL (0 °C), 24.75 g/100 mL (70 °C)
- Solubility in ethanol: 0.017 g/100 mL (16 °C)[5]
- Solubility in acetone: Insoluble
- Solubility in ether: Insoluble
Occurrences
Natural Occurrence: Barium Azide doesn’t naturally occur in the Earth’s crust. It is a synthetic compound produced through chemical reactions in labs or industrial settings.
Industrial Production: It is mainly produced by reacting sodium azide (NaN₃) with barium salts such as barium chloride (BaCl₂). The reaction yields barium azide as a precipitate:
2NaN₃ + BaCl₂ → Ba(N₃)₂ + 2NaCl
Uses
Barium azide can be used to make azides of magnesium, sodium, potassium, lithium, rubidium and zinc with their respective sulfates.
Ba(N3)2 + Li2SO4 → 2 LiN3 + BaSO4
It can also be used as a source for high purity nitrogen by heating:
Ba(N3)2 → Ba + 3 N2
This reaction liberates metallic barium, which is used as a getter in vacuum applications.
Barium Azide is not widely used in everyday products, but it does find applications in certain specialized fields, such as:
- Propellant systems and pyrotechnics: It can serve as a source of nitrogen gas, which is used in specific types of explosions or as part of an initiator for explosive devices.
- Rocketry and airbags: In the past, azides like barium azide were used in airbag systems or rocket propellants for their quick decomposition to generate gas.
Because of its hazardous nature, barium azide is not commonly found outside of specialized industrial environments.
Safety
Due to its explosive nature, it’s important to handle barium azide with extreme care, following safety protocols to avoid accidental detonation. It is typically stored and transported under controlled conditions.