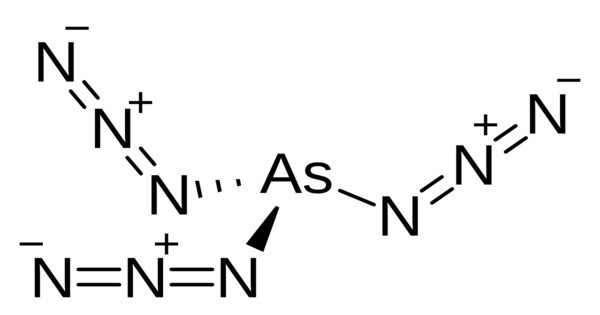
Arsenic triazide is an inorganic chemical compound with the formula As(N3)3. It is a highly reactive and toxic compound made of arsenic and azide groups. It is a toxic, shock-sensitive, and friction-sensitive white solid that melts at 37 °C and explodes on further heating. It is a white, crystalline substance that is sensitive to shock, heat, and friction, making it extremely hazardous to handle. Due to its toxicity and sensitivity, it has no commercial uses.
Arsenic triazide is primarily of interest in chemical and explosives research, due to its potential to be used as a detonating agent in explosives, although its dangerous instability limits its practical use.
Properties
- Chemical formula: As(N3)3
- Molar mass: 200.98 g/mol
- Appearance: White solid
- Density: 2.33 g/cm3
- Melting point: 37 °C (99 °F; 310 K)
- Boiling point: 62 °C (144 °F; 335 K) (decomposition)
- Solubility in water: Reacts
Synthesis and structure
Arsenic triazide was first synthesized by Thomas M. Klapötke in 1995 by the reaction of arsenic trichloride and sodium azide in trichlorofluoromethane at 0 °C:
AsCl3 + 3 NaN3 → As(N3)3 + 3 NaCl
A purer product was obtained by another synthesis route by Karl O. Christe in 2004 by the reaction of arsenic trifluoride and trimethylsilyl azide.
Both in the gas and solid phase, arsenic triazide adopts a trigonal pyramidal geometry around the arsenic atom with a bond angle of 88.3°; this low bond angle is attributed to the major p-character of the bonding orbitals. The point group is C3, meaning that the azide groups are not equivalent. However, in the solid phase, arsenic triazide attains a coordination number of 7, different from the gas phase, which has a coordination number of 4.
Reactivity
Due to the presence of azide groups, arsenic triazide is extremely sensitive to external stimuli such as heat, shock, and friction, which can lead to violent decomposition. This makes it very dangerous to work with.
Toxicity
Both arsenic and azide compounds are highly toxic. Arsenic is a potent poison that affects various organs, particularly the liver, kidneys, and nervous system. Azides can also be toxic, and exposure can lead to symptoms such as headache, dizziness, and even death in severe cases.
Uses
Due to its instability and high danger, arsenic triazide is not widely used in commercial applications. However, it has been studied in the context of explosives and as a potential detonator in military applications.
Safety
Handling arsenic triazide requires extreme caution. Proper safety protocols, including the use of protective clothing and equipment, should be followed. In addition, it should be stored and handled in controlled environments to minimize the risk of accidental detonation.