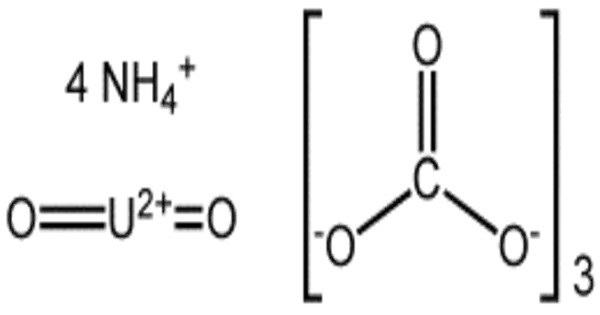
Ammonium uranyl carbonate (UO2CO3·2(NH4)2CO3) is known in the uranium processing industry as AUC and is also called uranyl ammonium carbonate. It is a yellow, crystalline compound used primarily in nuclear fuel production. This compound is important as a component in the conversion process of uranium hexafluoride (UF6) to uranium dioxide (UO2). It is a key intermediate in converting uranium ore into uranium dioxide (UO₂) or uranium hexafluoride (UF₆) for nuclear reactors.
The compound is sparingly soluble in water, stable under standard conditions, and forms through precipitation from uranyl nitrate solutions with ammonium carbonate. Its structure features a uranyl ion (UO₂²⁺) coordinated with three carbonate ligands, stabilized by ammonium ions. It is synthesized in industrial processes by treating uranium solutions with ammonium carbonate, followed by filtration and drying.
Properties
- Chemical formula: UO2CO3·2(NH4)2CO3
- Molar mass: 522.199 g/mol
- Appearance: lemon-yellow crystalline
- Density: 2.72
- Melting point: Decomposes between 165 °C and 185 °C
- Solubility in water: Insoluble
Natural Occurrence
Ammonium uranyl carbonate occurs in nature as the mineral liebigite, which is found in uranium deposits, particularly in oxidized zones of uranium ore bodies. Common in secondary uranium minerals formed through weathering or alteration of primary uranium minerals like uraninite (UO₂).
Synthetic Production
Ammonium uranyl carbonate is produced industrially during uranium processing, particularly in the refining of uranium ore to produce “yellowcake” (a mixture of uranium compounds, including ammonium diuranate and ammonium uranyl carbonate). It is formed by precipitating uranium from aqueous solutions using ammonium carbonate, a common step in uranium extraction and purification.
Applications
Applications include uranium enrichment and ceramic production. Its disposal follows strict regulations to minimize environmental impact. Research continues to optimize its synthesis for efficiency and safety in nuclear applications. The compound is radioactive due to uranium content, requiring careful handling to avoid health risks like kidney damage or cancer from prolonged exposure. It is non-flammable but must be stored securely to prevent environmental contamination.
Safety Considerations
- Radioactivity: Due to its uranium content, ammonium uranyl carbonate emits alpha and beta radiation, requiring strict handling protocols to minimize exposure.
- Toxicity: Uranium compounds are toxic, particularly affecting the kidneys. Inhalation or ingestion must be avoided.
- Environmental Impact: Improper disposal can lead to environmental contamination, especially in water systems, due to uranium’s radiological and chemical toxicity.