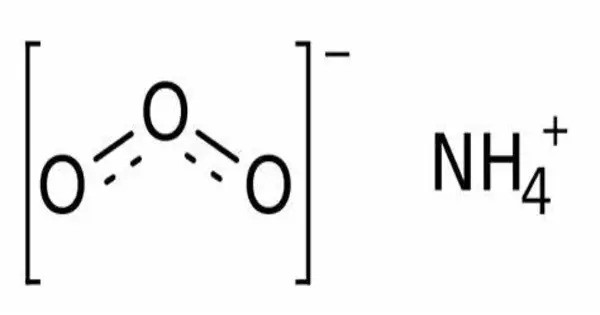Ammonium ozonide is an oxygen rich molecule containing an ammonium cation (NH4+) and an ozonide anion (O3−). Ammonium ozonide, like alkali ozonides, is a red solid. It’s quite unstable and is usually studied in controlled environments. It is stable at low temperatures, but it decomposes to ammonium nitrate at temperatures above -70 °C.
This compound doesn’t exist in a stable form for long periods, as it tends to decompose quickly, releasing oxygen and producing other byproducts. Due to the instability of ozone and its reactive nature, ammonium ozonide is primarily of interest in theoretical studies and in laboratory conditions for exploring reactions involving ozone.
Preparation and decomposition
Ammonium ozonide is made by bubbling gaseous ozone through liquid ammonia at -110 °C. This method suffers from a low yield.
12 NH3 + 11 O3 → 9 NH4O3 + 3 NO2
Ammonium ozonide decomposes into ammonium nitrate, oxygen gas, and water. If the above reaction is done at high temperatures, these decomposition products result immediately and no ozonide is formed.
4 NH4O3 → 2 NH4NO3 + O2 + 4 H2O
Properties
Ammonium ozonide is highly unstable, much like many other ozonides. This instability makes it prone to decomposition, which can result in the release of ozone gas (O₃) and nitrogen-containing products. Being an ozonide, it is reactive and sensitive to heat, light, and shock, which can trigger its decomposition.
- Chemical formula: H4NO3
- Molar mass: 66.036 g·mol−1
- Appearance: Deep red solid
Occurrences
Ammonium ozonide is not something typically found in nature or in large-scale chemical processes. It may form transiently in laboratory conditions, especially when ozone is bubbled through an ammonia solution under specific, controlled conditions. It is not commercially available, and its occurrence is mostly limited to experimental settings.
Applications
Due to its instability, ammonium ozonide doesn’t have significant practical applications. Its primary interest is in research related to ozone chemistry and reactive oxygen species, as ozonides can be important intermediates in these studies.