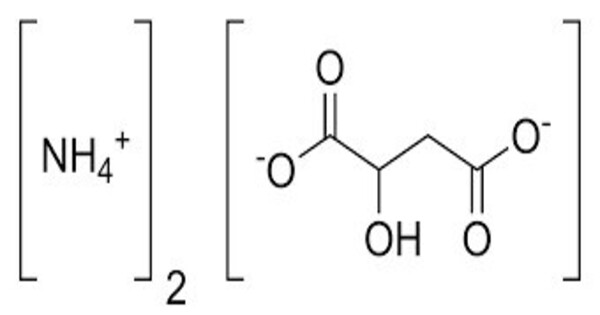
Ammonium malate refers to organic compounds containing malate and ammonium. It typically appears as a crystalline solid. Its exact appearance can vary depending on the specific form and purity. It is soluble in water, which is consistent with many ammonium salts and organic acids. In aqueous solution, it is generally slightly acidic due to the presence of malate ions and the weak acidity of malic acid. It is relatively stable under normal conditions but can decompose under extreme conditions or with prolonged exposure to moisture.
As a food additive, diammonium malate has been used as a flavoring agent and as an acidity regulator. It has the E number E349.
Properties
- Chemical formula: C4H9NO5
- Molar mass: 151.118 g·mol−1
- Appearance: white solid
- Density: 1.498 g/cm3 (monohydrate)
Occurrences
- Biological Context: Ammonium malate is not commonly found in large quantities in nature. However, malates and ammonium ions are important in biological systems. Malic acid, from which ammonium malate is derived, is a key component of the citric acid cycle (Krebs cycle) in cellular respiration.
- Synthetic Context: It can be synthesized in the laboratory by reacting malic acid with ammonia. This synthetic route is useful for studying its properties or for use in specific applications.
Applications
- Agricultural and Industrial Uses: Although not widely used, ammonium malate may be used in niche applications such as in certain formulations of fertilizers or in the production of other chemicals.
- In Food and Beverages: Malic acid, a parent compound, is used in the food industry as an acidulant, but ammonium malate itself is not commonly used in food products.