Ammonium azide is the chemical compound with the formula [NH4]N3, being the salt of ammonia and hydrazoic acid. It’s a colorless, crystalline substance that is highly sensitive and can be dangerous due to its instability, especially when subjected to heat, shock, or friction. Like other inorganic azides, this colourless crystalline salt is a powerful explosive, although it has a remarkably low sensitivity.
It’s often used in the production of airbags in vehicles because it decomposes rapidly into nitrogen gas when ignited, creating a rapid inflating effect. It is physiologically active and inhalation of small amounts causes headaches and palpitations. It was first obtained by Theodor Curtius in 1890, along with other azides.
Structure
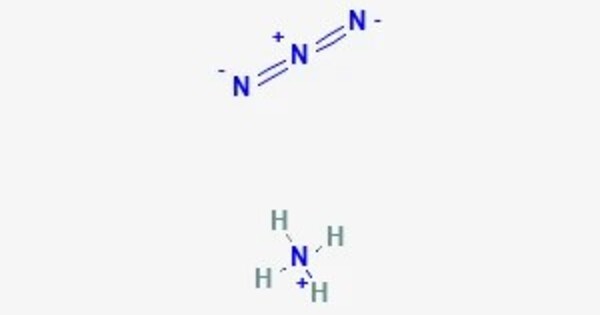
Ammonium azide is ionic, meaning it is a salt consisting of ammonium cations [NH4]+ and azide anions N−3, therefore its formula is [NH4]+[N3]−. It is a structural isomer of tetrazene. Ammonium azide contains about 93% nitrogen by mass.
Properties
Ammonium azide typically appears as a white crystalline solid. It is soluble in water, and this solubility increases with temperature. It is relatively unstable and can be sensitive to heat, shock, or friction, making it a dangerous compound to handle. It can decompose explosively under certain conditions.
- Chemical formula: [NH4]N3
- Molar mass: 60.060 g·mol−1
- Appearance: Colorless or white crystalline solid
- Odor: Odorless
- Density: 1.3459 g/cm3
- Melting point: 160 °C (320 °F; 433 K)
- Boiling point: 400 °C (752 °F; 673 K) (decomposes)
- Crystal structure: Orthorhombic
Occurrences
Ammonium azide doesn’t naturally occur in large quantities in nature. However, it is primarily synthetically produced, and it has applications in various fields:
- Explosives: Due to its sensitivity and explosive properties, ammonium azide is used in some types of pyrotechnics and as a detonator in automotive airbags.
- Laboratory Synthesis: In research, it might be synthesized for specific chemical reactions, particularly those requiring the release of nitrogen gas.
- Airbags: It is used in automotive airbag systems, where it quickly decomposes to generate nitrogen gas, inflating the airbag during an accident.
Safety Considerations
Given its sensitivity, ammonium azide requires careful handling, especially in industrial and laboratory settings. It’s typically stored under controlled conditions, away from heat and shock sources. Because of its potential hazards, ammonium azide must be handled with extreme care and is typically not used outside of controlled environments.