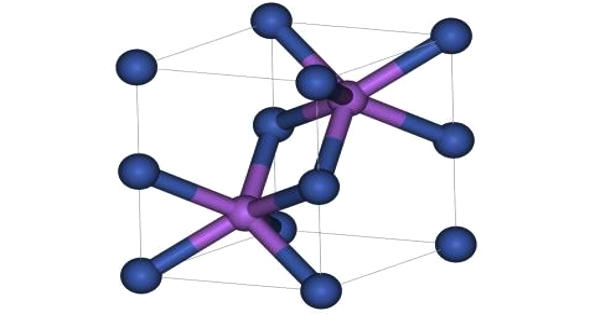
Aluminium selenide (Al₂Se₃) is an inorganic compound composed of aluminium and selenium. It appears as a pale yellow to gray solid and crystallizes in a structure similar to aluminium oxide, with aluminium ions surrounded by selenium atoms. The compound is highly sensitive to moisture and reacts vigorously with water, producing toxic hydrogen selenide (H₂Se) gas, which has a foul odor and poses significant health hazards. For this reason, it must be handled in dry, controlled environments.
Al₂Se₃ is typically synthesized by direct combination of elemental aluminium and selenium at elevated temperatures. It is classified as an ionic semiconductor, with applications in optoelectronics, infrared technologies, and as a precursor material for preparing other selenium-containing compounds. Due to its semiconducting nature, it is studied for potential use in photovoltaic cells and electronic devices.
Properties
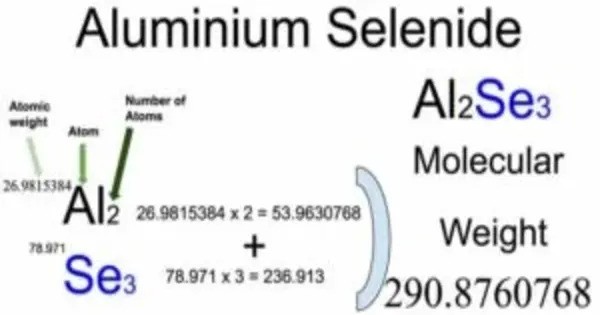
- Chemical formula: Al2Se3
- Molar mass: 290.84 g/mol
- Appearance: yellow to brown powder
- Odor: odorless
- Density: 3.437 g/cm3
- Melting point: 947 °C (1,737 °F; 1,220 K)
- Solubility in water: decomposes
Preparation
It is a solid prepared by igniting a mixture of the elements at 1,000 °C (1,830 °F):
2 Al + 3 Se → Al2Se3
The pure compound is white, but typical samples are coloured. Samples are usually protected from moisture, because they hydrolyze readily, giving off highly toxic hydrogen selenide gas:
Al2Se3 + 3 H2O → Al2O3 + 3 H2Se
Occurrences
Aluminium selenide does not occur naturally due to its reactivity with water and oxygen. It is produced synthetically in laboratories or industries by direct combination of aluminium and selenium at high temperature under an inert atmosphere. Its significance lies mainly in research and niche semiconductor uses, rather than in naturally found minerals.
Use
Al2Se3 has been used as a precursor to hydrogen selenide, which is released when the solid is treated with acids. Al₂Se₃ reacts with water or acids to produce H₂Se gas, which is highly toxic but essential in semiconductor and photovoltaic industries.
Safety
The material is stable under dry, inert conditions but decomposes in humid air. In terms of safety, exposure to hydrogen selenide released from hydrolysis can cause severe irritation, respiratory damage, and even fatal poisoning. Proper protective measures and inert atmosphere storage are essential when working with aluminium selenide. Overall, it is a valuable but hazardous compound, mainly of interest in research and specialized industrial applications.