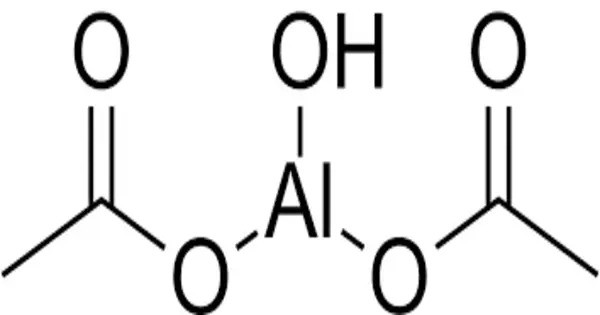
Aluminium diacetate, also known as basic aluminium acetate, is a white powder with the chemical formula C4H7AlO5. It consists of aluminum (Al) and two acetate ions (CH₃COO⁻). It is sometimes used as a precursor in various chemical syntheses or as a catalyst in certain reactions. It is one of a number of aluminium acetates and can be prepared in a reaction of sodium aluminate (NaAlO2) with acetic acid. The compound has applications in both industrial and laboratory settings.
In its pure form, aluminum diacetate is typically a white crystalline solid, but it is rarely encountered outside of controlled settings because it’s usually involved in specific chemical processes. The compound may also be used in small-scale chemical reactions or may be part of a solution for various applications.
Properties
- Chemical formula: C4H7AlO5
- Molar mass: 162.077 g·mol−1
- Appearance: White, opaque crystals
- Solubility: It is soluble in water and in some polar solvents. The solubility in water is due to the ionic nature of the compound, where it dissociates into its constituent ions.
- Hygroscopic: It tends to absorb moisture from the air, making it hygroscopic.
- Stability: This compound is generally stable under normal conditions. However, when exposed to excess moisture or high temperatures, the compound may decompose.
- Chemical Reactivity: It is a salt that may react with strong acids to release acetic acid and form other aluminium compounds. It may also decompose when exposed to high temperatures.
Synthetic Preparation
Aluminium Diacetate is typically prepared in laboratories or industrial settings through the reaction between acetic acid and aluminium salts. One common method involves reacting aluminium hydroxide (Al(OH)₃) with acetic acid.
In Nature
Aluminium Diacetate is not commonly found as a natural mineral. However, aluminium salts in various forms (such as aluminium sulfate, chloride, or hydroxide) can be found in nature, but aluminium diacetate itself does not naturally occur in large quantities or in significant mineral forms.
Industrial Use
Aluminium Diacetate is used in a variety of applications, including in the textile industry, as a mordant, or as a catalyst in certain chemical reactions. It may also be used in cosmetic formulations and in the food industry in trace amounts, though its presence is not as common as other aluminium salts like aluminium chloride or aluminium sulfate.
In Medicine and Cosmetics
Aluminium diacetate is used as an antiseptic and astringent. It is used topically as wet dressing, compress, or soak for self-medication to temporarily relieve itching and soothe, particularly on wet or weeping lesions. It relieves skin irritation from many causes, such as insect bites, athlete’s foot, urushiol-induced contact dermatitis from plants poisonous to the touch such as poison ivy, oak, or sumac, and skin irritation due to sensitivity to soaps, detergents, cosmetics, or jewellery. It is also used to relieve swelling from bruises.
Some aluminium salts, including aluminium diacetate, have been researched for use in topical treatments or as a component in certain types of ointments or products, though this compound is less prevalent than other aluminium compounds like aluminium acetate (which is used for skin irritation).