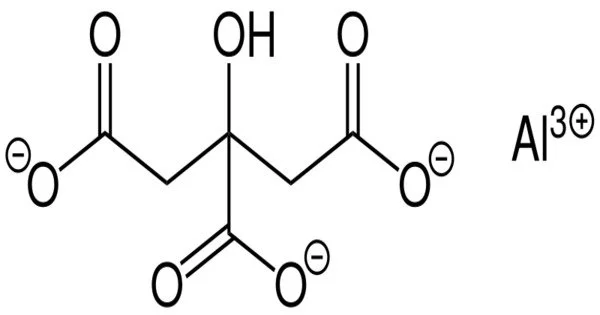
Aluminium citrate is a chemical compound with the chemical formula AlC6H5O7. It can exist in various forms, often as a salt of aluminum and citric acid. This white, crystalline salt is produced by mixing aluminium chloride hexahydrate and citric acid. It can be formed by the reaction between aluminium salts (such as aluminium chloride) and citric acid.
Properties
- Chemical formula: AlC6H5O7
- Molar mass: 216.08 g/mol
- Appearance: White solid
- Solubility in water: Insoluble
- Stability: The compound is stable under normal conditions, though it is hygroscopic (absorbs moisture from the air).
Occurrences
Natural Occurrence: Aluminium citrate does not occur naturally in large quantities but may form in certain biological systems or under conditions where aluminium salts and citric acid are present. For instance, it might form as a result of natural processes involving the decomposition of organic materials in soils or water systems rich in aluminium and organic acids (such as citric acid).
In Biological Systems: Citric acid is a naturally occurring compound in all living organisms as part of the citric acid cycle (Krebs cycle), which is central to metabolism. While aluminium citrate itself isn’t typically found in large amounts in nature, its constituent elements (aluminium and citrate) might be found in various combinations within living organisms, especially in areas where aluminium accumulates in soil and water.
Uses
Aluminium citrate can be used as a crosslinker for many polymers in the oil industry. It is also used as an antiperspirant.
- In medicine: It’s sometimes used in certain formulations, especially for its ability to chelate metals. For example, it may be used in the treatment of certain conditions involving metal toxicity, as it can help bind metals like aluminum or other elements to facilitate their removal from the body.
- In chemistry and laboratories: It can be used as a reagent in some chemical analyses or reactions involving aluminum and citric acid.
- Potential effects: While aluminum salts like aluminum citrate have been investigated for use in reducing metal toxicity, aluminum compounds, in general, have been under scrutiny for their possible connection to health concerns, particularly neurological issues.
Effects on humans
Aluminium citrate takes up about 8% of aluminium in blood due to the ability of Al3+ ions to replace Ca2+ from calcium citrate and is known to cause chronic renal failure because it causes an increase of phosphorus in the kidneys. It has been suspected to cause Alzheimer’s disease but more evidence is needed. This compound can also have some positive effects on humans such as preventing silicosis. When ingested, 80% of the compound is excreted through the body through urine and the rest comes out slower.