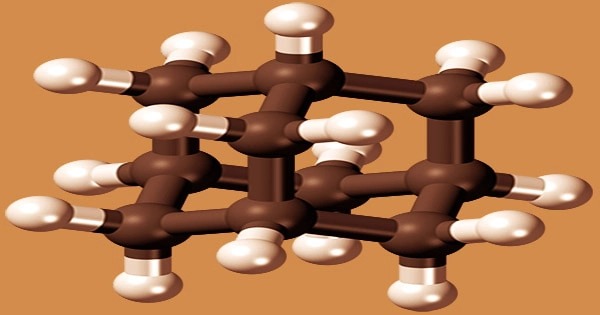
Adamantane is a polycyclic alkane that belongs to the adamantanes family. It is also a tricyclic aliphatic hydrocarbon that belongs to the cluster-like compound family and is found naturally in petroleum with a concentration of roughly four millionths. It’s an organic chemical with the formula C10H16, or (CH)4(CH2)6 to be more precise. It is made by reacting dicyclopentadiene with hydrogen in a nickel catalyst and an aluminum trichloride catalyst.
Adamantane molecules are made up of three cyclohexane rings fused together; the molecule is stiff and essentially stress-free. It’s a cyclic tetrahedral arrangement with 10 carbon atoms and 16 hydrogen atoms that forms a cage-like compound with good symmetry. Its derived notoriety name adamantane comes from the fact that its spatial arrangement of carbon atoms is typically the same as the fundamental unit of the diamond lattice.
As the simplest of the so-called diamondoids molecules that combine adamantane-like carbon cages, adamantane is structurally fascinating, but it isn’t especially useful. The spatial arrangement of carbon atoms in the adamantane molecule is the same as in the diamond crystal, making it the most stable isomer of C10H16. However, adamantane can be used as a starting point for the creation of bigger compounds, such as pharmaceuticals.
High thermal stability, good lubricity, high pro-oil capacity, no flavor, and lower reactivity than benzene are all characteristics of adamantane. It may also be oxidized to generate diamond alcohol and utilized to make speciality polymers, such as optical and photosensitive materials; it can also be used to make gasoline, as well as co-catalysts, lubricants, and pharmaceuticals. In 1933, the discovery of adamantane in petroleum sparked a new branch of chemistry dedicated to the synthesis and characteristics of polyhedral organic molecules.
Landa et al (Czech) found adamantane from petroleum fractions at the South Moravia oilfield in 1932. In the next year, he used X-ray technology to validate the structure of the object. Drugs, polymeric materials, and thermally stable lubricants are all examples of adamantane derivatives in use. During his time in Switzerland in 1941, scientist Vladimir Prelog (Yugoslavia) was the first to effectively synthesize it. It was synthesized at the time using a twenty-step stepwise synthesis process. At the time, adamantane was a somewhat pricey chemical.
Starting in 1967 with adamantadine, which is just adamantane with one hydrogen substituted by ammonia, a wide series of compounds based on the adamantane structure have been developed. As a result of the peculiar cage structure of adamantane piqued the interest of the scientific world, the science of caged compound chemistry was born. Amantadine was later shown to have antiviral activity, which drew even more interest from the medical community.
Adamantane is sublimed in a quartz tube that is put in a furnace with numerous heaters that maintain a temperature gradient along the tube (approximately 10 °C/cm for adamantane). Crystallization begins at one end of the tube, which is held at the adamantane freezing point. The quantity of adamantane in petroleum is around four millionths of a percent. It’s made by catalyzing the hydrogenation of dicyclopentadiene to tetrahydro-dicyclopentadiene, then isomerizing it in the presence of anhydrous aluminum chloride.
The concentration of adamantane in petroleum varies from 0.0001 percent to 0.03 percent depending on the oil field and is too low for practical production. Adamantane is a tetrahedral cyclic hydrocarbon having 10 carbon atoms and 16 hydrogen atoms, with the chair form of cyclohexane as its primary structure. For a hydrocarbon, it has a very high melting point. Its melting point of 270 °C is substantially higher than that of other hydrocarbons with the same molecular weight, such as camphene (45 °C), limonene (74 °C), ocimene (50 °C), terpinene (60 °C), or twistane (164 °C), as well as that of the linear C10H22 hydrocarbon decane (28 °C).
Adamantane is primarily utilized in the production of anti-cancer and anti-tumor medications. It may also be utilized to make improved lubricants, photosensitive surfactants, insecticides, catalysts, and other materials. The nitration of adamantane is a challenging process with low yields. By reacting adamantane with bromine or nitric acid to produce the bromide or nitroester at the 1-position, the nitrogen-substituted medicine amantadine can be made.
In the presence of anhydrous aluminum chloride, adamantane may also be produced by isomerizing tetrahydro-dicyclopentadiene. Its derivatives, such as 1-amino-adamantane hydrochloride and 1-adamantyl triethylamine hydrochloride, can be used as medicines to prevent influenza caused by the virus A2. Because its absorption bands are in the vacuum-ultraviolet area of the spectrum, adamantane can be used to extend the life of the gain medium in dye lasers. It cannot be photoionized under atmosphere because its absorption bands are in the vacuum-ultraviolet region of the spectrum. It’s frequently utilized as a pharmaceutical intermediary, as well as a raw ingredient for light-sensitive materials, cosmetic and surfactant intermediates, and epoxy curing agents.
Information Sources: