Actinium oxychloride or actinium oxide chloride is an inorganic compound of actinium, oxygen, and chlorine with the chemical formula AcOCl. It is composed of actinium, oxygen, and chlorine. This compound is part of the actinide oxyhalide family, typically formed when actinium chloride undergoes partial hydrolysis. Actinium oxychloride appears as a white crystalline solid, though it is rarely observed due to the extreme radioactivity of actinium and its scarcity in nature.
It is generally prepared by gently heating actinium chloride (AcCl₃) in a moist environment or by controlled hydrolysis of actinium hydroxide in hydrochloric acid vapors. Actinium oxychloride is insoluble in water but can dissolve in concentrated acids. Like most actinium compounds, it exhibits radioactive properties, primarily emitting alpha radiation.
Properties
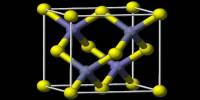
The compound forms white crystals of the tetragonal system, structure type of PbClF. It is typically formed as a white or slightly off-white solid. The compound is slightly soluble in water and more soluble in acidic solutions. It is known for being radioactive, as it contains actinium, a member of the actinide series with strong alpha-emitting properties. It is thermally stable up to moderate temperatures but decomposes at high heat. It can be obtained by partially hydrolyzing actinium trichloride (AcCl₃) or by reacting actinium oxide with hydrochloric acid under controlled conditions.
- Chemical formula: AcOCl
- Molar mass: 278 g/mol
- Appearance: white crystals
- Density: 7.23 g/cm3
- Melting point: 1,000 °C (1,830 °F; 1,270 K)
Chemically, Actinium Oxychloride behaves similarly to lanthanum oxychloride (LaOCl) because of their comparable ionic radii and oxidation states (+3). It is thermally stable up to moderate temperatures but decomposes upon strong heating to actinium oxide and chlorine gas.
Synthesis
AcOCl can be synthesized by reacting AcCl3 with water at 1000 °C. Also, it can be produced by heating AcCl3 in ammonia vapor at 900 °C.
Occurrences
Actinium oxychloride does not occur naturally in the environment. It is produced in laboratories as an intermediate during the preparation and study of actinium compounds. Actinium itself is found in trace amounts in uranium and thorium ores, such as pitchblende, from which it is isolated. Actinium oxychloride is mainly of interest for research purposes in radiochemistry and nuclear science due to its radioactivity and rarity.



![Report on Overall Banking Practice of National credit and commerce bank [Part-2]](https://assignmentpoint.com/wp-content/uploads/2013/05/images-11-110x55.jpg)