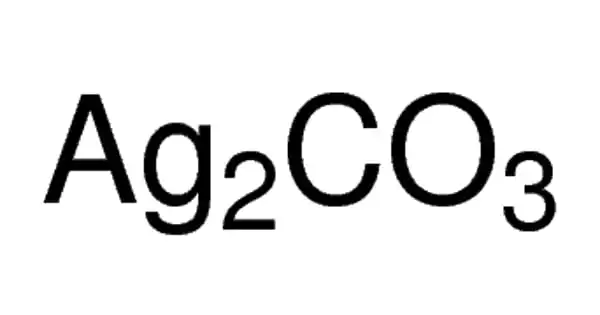The chemical compound with the formula Ag2CO3 is silver carbonate. It is a colorless yellow to brown solid with no odor. Although this salt is yellow, most samples are grayish due to the presence of elemental silver. It is a chemical compound that is extremely reactive and unstable. Like most transition metal carbonates, it is poorly soluble in water.
Silver carbonate is essential in microelectronics. It aids in the production of silver powder, which is widely used in the chip industry. It has high alkynophilicity and basicity, making it an excellent catalyst in a variety of organic reactions.
Physical Properties –
The molecular weight of silver carbonate is 275.7 g/mol. It has a melting point of 424 degrees Fahrenheit (218 degrees Celsius), indicating that it is most commonly found as a solid. But it decomposes, or the bonds break apart, at 248 degrees Fahrenheit (120 degrees Celsius), a long time before it melts. It crystallizes into light-yellow crystals.
- Melting point: 210 °C (dec.) (lit.)
- Density: 6.08 g/mL at 25 °C (lit.)
- Storage temp.: Keep in dark place,Inert atmosphere,Room temperature
- Form: Granular Powder
- Color: Green-yellow to greenish
- Specific Gravity: 6.08
- Water Solubility: insoluble

Preparation and reactions
Silver carbonate can be prepared by combining aqueous solutions of sodium carbonate with a deficiency of silver nitrate.
2 AgNO3(aq) + Na2CO3(aq) → Ag2CO3(s) + 2 NaNO3(aq)
Freshly prepared silver carbonate is colourless, but the solid quickly turns yellow.
When silver carbonate reacts with ammonia, the complex ion diamminesilver(I) ([Ag(NH3)2]+) is formed. There is a possibility that explosive Silver nitride will precipitate out of the solution, as with other diamminesilver(I) solutions, including Tollen’s reagent. The IUPAC has discontinued the use of silver nitride, which was previously known as fulminating silver due to confusion with silver fulminate.
With hydrofluoric acid, it gives silver fluoride.
The thermal conversion of silver carbonate to silver metal proceeds via the formation of silver oxide:
Ag2CO3 → Ag2O + CO2
2 Ag2O → 4 Ag + O2
Structure
The bonds formed in this compound are the result of several factors, the most important of which is the molecular mass of silver carbonate. To achieve stability, the atoms arrange themselves as shown below. Nonetheless, silver carbonate is a highly unstable compound.
Uses
Silver carbonate is primarily used to make silver powder for use in microelectronics. It is reduced with formaldehyde to produce silver that is devoid of alkali metals:
Ag2CO3 + CH2O → 2 Ag + 2 CO2 + H2O
Ag2CO3 can be used as a base for the Pd-catalyzed oxyarylation of olefins by ortho-iodophenols. It can also be used for Koenigs-Knorr glycosylation. It is used as a reagent in organic synthesis reactions such as the Koenigs-Knorr reaction. Silver carbonate on celite is used as an oxidizing agent in the Fétizon oxidation to form lactones from diols. It is also used to convert alkyl bromides into alcohol. It has been used as a base in the Wittig reaction and in C-H bond activation.
It acts as a reagent in a critical organic synthesis reaction known as the Koenig – Knorr reaction. The compound is important in the electronics industry because it produces silver, which is used in chips and microprocessors.