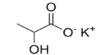
Magnesium sulfate (MgSO4) appears as white or colorless needle-like or inclined columnar crystal, being odorless, consisting of magnesium cations Mg2+ (20.19% by mass) and sulfate anions SO2−4. It is a magnesium salt with counterion sulfate and water-soluble, but not ethanol. With a molecular weight of 246.47, specific gravity 1.68, it is mild and slightly salty, being easily soluble in water, and partially soluble in ethanol and glycerol.
Magnesium sulfate plays a role as an anticonvulsant, a cardiovascular drug, a blocker of the calcium channel, an anesthetic, a tocolytic agent, an antiarrhythmic medication, an analgesic, and an engrais. It is usually encountered in the form of a hydrate MgSO4·nH2O, for various values of n between 1 and 11. In 67.5 °C, it is soluble in its own crystal water. It may be subject to thermal decomposition at 70, 80 ° C, with the loss of four crystal water molecules. To become an anhydrous product it will lose all of the crystalline water at 200 ° C.
The most common is the heptahydrate MgSO4·7H2O, known as Epsom salt, which is a household chemical with many traditional uses, including bath salts. This is a salt of magnesium, and sulfate of copper. Through air (dry), it is easy to weather into powder with heating that slowly eliminates the molecules of crystal water to form anhydrous magnesium sulfate. The heptahydrate derives its common name from a bitter saline spring in Epsom in Surrey, England, where the salt was extracted from the springs that originate from where the North Downs porous chalk meets non-porous clay in London.
Despite being useful despite quantitative monitoring in manufacturing, magnesium sulfate heptahydrate is difficult to dissolve and thus easier to measure than anhydrous magnesium sulfate. For agriculture, the key use of magnesium sulfate is to correct magnesium deficient soils (an important plant nutrient). A thin, colorless crystal used in the treatment of preeclampsia and eclampsia as an anticonvulsant, a cathartic, and an electrolyte replenisher. The monohydrate is favored for this use; by the mid-1970s, its production was 2.3 million tons annually. It is used for printing fine cotton, silk because of the weighting agent of cotton yarn and also the filler of kapok products; as Epsom salt of drugs.
Magnesium is the core element of chlorophyll, enabling plant reduction processes to be enhanced and disease resistance to improve; it is essential for photosynthesis. Throughout nature, the anhydrous phase and some hydrates exist as salts, and the salt is a major component of some springs’ water. Magnesium sulfate is a very effective fertilizer, as it can provide rich nutrition for the crop. Magnesium has proven to be a significant source of increased productivity and agricultural growth and must be replenished in a timely manner because excessive extraction of magnesium from the soil is the main cause of this widespread disease. This can be combined according to specific needs with nitrogen, phosphorus, and potassium into compound fertilizer or combined fertilizer, and can be blended into various fertilizers and photosynthetic fertilizers with one or more elements respectively. After the contrasting practical fertilization experiment treating nine crops including rubber trees, fruit trees, tobacco leaves, potatoes and cereals, magnesium-containing compound fertilizer that make the crop grow 15-50 percent better than magnesium-free fertilizer.
In geological environments, magnesium sulfates are rising minerals. An incidence is often related to processes of the supergenes. Many of them are also essential constituents of salt deposits containing evaporative potassium-magnesium (K-Mg). Magnesium is that the activator of varied enzymes in livestock and poultry, and plays an especially important role in animal metabolism and nerve function. If the animal body undergoes magnesium deficiency, it’ll cause material metabolism and neurological disorders, supply disorders, affecting the expansion and development of livestock and poultry, and even result in death.
Magnesium sulfates are used in the diagnosis of serious pregnancy toxemias (pre-eclampsia and eclampsia) and in the treatment of acute nephritis in children for the rapid management of life-threatening convulsions. Also suggested for magnesium deficiency replacement therapy, especially in acute hypomagnesemia accompanied by tetany signs similar to those of hypocalcemia. It is also employed in uterine tetany as a myometrial relaxant. Additionally, magnesium sulfate heptahydrate is accustomed to preserve the concentration of magnesium in marine aquaria containing large amounts of stony corals as it is gradually extracted in their calcification cycle.
Information Sources: