Acrylic Acid
Definition
Acrylic acid may be a colorless, corrosive liquid that readily forms polymers. It’s accustomed to make plastics, paints, synthetic rubbers, and textiles. It’s the only unsaturated acid, consisting of a group connected on to an acid terminus. The formula of Acrylic acid is: CH2=CHCOOH.

It is miscible with water, alcohols, ethers, and chloroform. More than a million tons are produced annually.
Production and Manufacturing Process of Acrylic acid
Acrylic acid is produced from propylene which is a byproduct of ethylene and gasoline production. It became commercially available when routes based on the reaction of acetylene with water and carbon monoxide, or an alcohol and carbon monoxide to give acrylic acid, were developed. Another early process was the reaction of ketene, obtained by the pyrolysis of acetone or acetic acid, with formaldehyde.
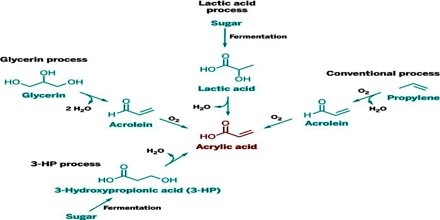
Acrylic acid and its esters have long been valued commercially, many other methods have been developed but most have been abandoned for economic or environmental reasons. An early method was the hydrocarboxylation of acetylene:
HCCH + CO + H2O → CH2=CHCO2H
This method requires nickel carbonyl and high pressures of carbon monoxide. It was once manufactured by the hydrolysis of acrylonitrile which is derived from propene by ammoxidation, but was abandoned because the method cogenerates ammonium derivatives. Other now abandoned precursors to acrylic acid include ethenone and ethylene cyanohydrin.
The oxidation of propylene produces acrolein, acrylic acid, acetaldehyde, and carbon oxides. Acrylic acid from the primary oxidation can be recovered while the acrolein is fed to a second step to make acrylic acid. Purification can be carried out by azeotropic distillation.

Uses of Acrylic Acid
The worldwide production of acrylic acid in 1994 was estimated to be approximately 2 million tonnes. Acrylic acid is used primarily as a starting material in the production of acrylic esters; as a monomer for polyacrylic acid and salts, as a comonomer with acrylamide for polymers used as flocculants, with ethylene for ion exchange resin polymers, with methyl ester for polymers.