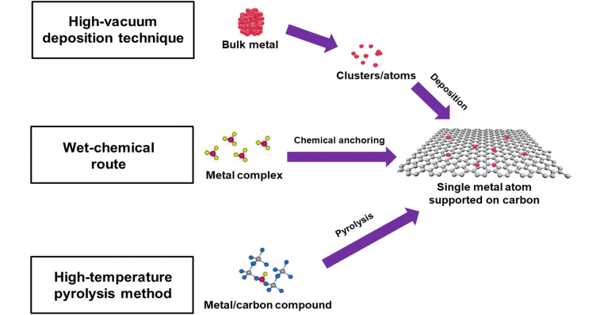
Single-atom catalysts (SAC) have recently emerged as a rising star in catalysis because they combine the benefits of both heterogeneous and homogeneous catalysts while bridging the gap with unique features. “Never change a running system,” as the saying goes. New methods, on the other hand, can be far superior to older ones. Currently, chemical reactions are primarily accelerated by catalytic materials made up of hundreds of atoms; however, the use of single atoms could provide a new approach to catalysis.
An international research team led by the TU Wien in Austria has created a new method for anchoring individual atoms on surfaces in a controlled and stable manner. This is a significant step toward single-atom catalysis. The new method was presented in the scientific journal ACS Nano by the researchers working with Bernhard C. Bayer.
Atom efficiency and specific activity (i.e., activity per atom) generally increase with decreasing particle size, with a single atom being the ultimate limit of size reduction. Dr. Liu’s method involves anchoring the single atom onto nanostructured supports, which immobilizes it and prevents it from clustering together. The findings of this study are guiding the molecular design of next-generation catalysts for energy and environmental applications.
The researcher developed a new method for anchoring individual atoms in a controlled and stable manner on surfaces. This is an important step towards single-atom catalysis.
Single atoms to replace nanoparticles
Long before this concept was extended to widely used heterogeneous catalysts based on supported metals, the isolation of elements as atoms is chemically distinct substances that played fundamental catalytic roles, for example, in metalloenzymes, organometallic complexes, and open framework structures.
Because modern catalysts are made of nanoparticles, they are extremely small. However, when measured on an atomic scale, they still have hundreds of atoms, making them far larger than single-atom catalysts. If single atoms can be used to accelerate chemical reactions, it could open up new avenues for catalysis. Single-atom catalysis can be more sustainable and energy-efficient than traditional processes, as well as more selective and achieve a higher turnover.
Silicon atoms act as “anchors” for single metal atoms in the newly developed method. Silicon atoms are frequently found as an impurity in carbon support materials. Indium atoms are now bound to these silicon atoms, and they can act as single-atom catalysts. “The indium atoms bind selectively to the silicon anchors in the carbon crystal lattice,” says Bernhard C. Bayer of the TU Wien’s Institute for Materials Chemistry.
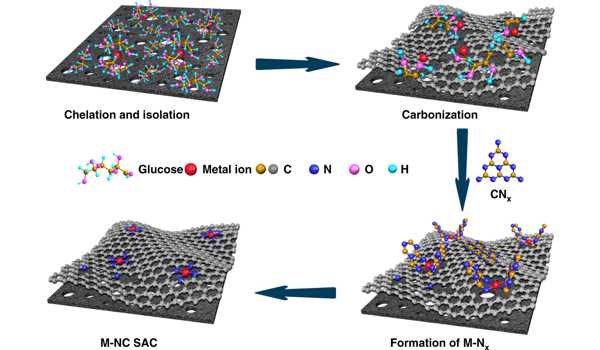
“As a result, the individual indium atoms remain stable and anchored at their positions and do not clump together,” says Bayer, the study’s lead author. “The new technology is especially exciting because the indium atoms are anchored in a self-assembled fashion if the reaction conditions are favorable. As a result, the process has the potential to be scalable “Kenan Elibol, the study’s first author and a professor at the University of Vienna and Trinity College Dublin.
The process, however, was not without its difficulties, which the research team overcame. Individual atom deposition on solid support surfaces, in particular, is difficult. This is due to the fact that single atoms normally move away from their locations quickly and clump together to form larger particles. The formation of such larger particles nullifies the benefits of single-atom catalysis.
Further tests to follow
The mechanisms of the silicon-anchoring of the indium single atoms were observed using a high-resolution electron microscope at the University of Vienna. “We were able to demonstrate that the anchoring of the indium atoms is dependent on how the silicon anchors are bound into the carbon crystal lattice,” says Toma Susi of the University of Vienna, who used modern computational methods to further elucidate the anchor structures.
“Such controlled and room-temperature-stable anchoring of individual atoms on solid surfaces has not previously been reported, and opens up exciting perspectives for catalytic applications in the fields of energy and environment,” says Dominik Eder, a catalysis expert at TU Wien.
Further experiments will be carried out so that the Viennese researchers’ method can be used in industry: “The single atoms placed with the new method are now to be tested in detail as catalysts for various chemical reactions,” says Bernhard C. Bayer.