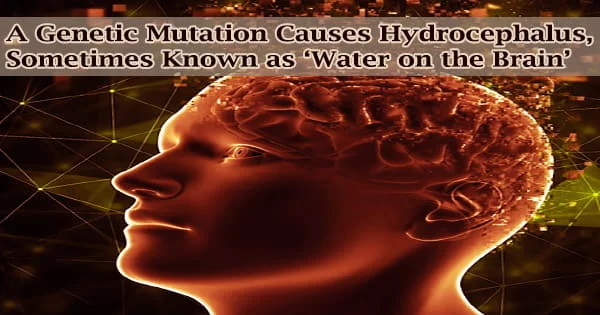
The cerebral ventricles, four interconnected chambers of the brain that are filled with cerebrospinal fluid, become enlarged in hydrocephalus, or “water on the brain,” but the cause is unknown in many cases.
Improved therapies for hydrocephalus, which is the main cause of brain surgery in children and is linked to neurodevelopmental impairment, could result from a better understanding.
A team lead by researchers from Massachusetts General Hospital (MGH) and Yale University looked into changes in genetic sequences and gene expression patterns in the brains of patients with congenital hydrocephalus to gain insight.
The findings, which were published in Nature Neuroscience, suggest that hydrocephalus is caused by primitive cells in the brain not behaving properly throughout development, rather than a problem with cerebrospinal fluid “plumbing.”
Continued accumulation of fluid dilates the cerebral ventricles, increases pressure in the skull, and compresses the surrounding brain structure in patients with hydrocephalus. This compression might result in immediate symptoms including vomiting and headaches, as well as coma or death.
Even when a medical device called a shunt is surgically implanted in the brain, long-term brain compression can cause neurocognitive difficulties and neurodevelopmental disorders in children.
“Neurosurgical shunting of cerebrospinal fluid addresses some consequences of the disease but does not target the underlying mechanisms,” says senior author Kristopher T. Kahle, MD, PhD, director of Pediatric Neurosurgery at MGH and director of the Harvard Center for Hydrocephalus and Neurodevelopmental. “Knowing the molecular cause of disease could be very helpful towards clinical decision making.”
To gain insight, Kahle and his colleagues used a profiling approach that detects gene abnormalities in patients throughout the entire genome to genetically sequence cells from 483 children with hydrocephalus and their unaffected parents.
In the long-term, with continued gene discovery and better understanding of how other gene mutations disrupt brain development to cause hydrocephalus, we may be able to develop drug treatments or even gene therapy to correct the gene mutations months before the birth of patients.
Kristopher T. Kahle
The team discovered that many hydrocephalus-associated genes converge in neuroepithelial cells, which are the earliest stem cells in the brain that arise during the first few weeks of development, rather than in fluid circulation components, by combining genetic sequence data with gene expression data. These cells go on to produce all of the brain’s neurons and support cells.
“This began to hint to us that rather than affecting fluid circulation, hydrocephalus gene mutations may be disrupting the earliest processes of human brain development to cause hydrocephalus,” says co-lead author Phan Q. Duy, an MD/Ph.D. student at Yale University School of Medicine.
TRIM71, the most frequently altered gene in the study’s patients, codes for a protein that is part of a pathway that controls stem cell development time. When the researchers produced mice with TRIM71 mutations, the mice developed fetal-onset hydrocephalus in the same way as humans do.
The Trim71-mutated mice’s brain stem cells prematurely produced neurons, resulting in an inadequate pool of stem cells to sustain brain growth and development. This resulted in a lack of brain tissue expansion and cerebral cortex underdevelopment.
According to the researchers, the brain’s changed structure is unable to withstand the pressure exerted by cerebrospinal fluid, causing the brain to deform and its ventricles to passively enlarge.
“The site of pathology is therefore not happening in the fluid itself, but rather the vessel or the brain tissue that’s holding the fluid,” says Duy.
The findings suggest that treatment strategies for hydrocephalus should go beyond draining fluid in the brain.
“A more nuanced treatment approach may include not only cerebrospinal fluid diversion but also other approaches more tailored towards improving neurodevelopmental function,” says Kahle.
“In the long-term, with continued gene discovery and better understanding of how other gene mutations disrupt brain development to cause hydrocephalus, we may be able to develop drug treatments or even gene therapy to correct the gene mutations months before the birth of patients.”
This research could lead to new insights into other pediatric brain illnesses, in addition to a better knowledge of hydrocephalus. Many of the processes associated in hydrocephalus may also be relevant for other structural brain defects, as ventricular dilatation is a prevalent feature in developmental neuropsychiatric illnesses like autism and schizophrenia.
The National Institutes of Health, the Rudi Schulte Institute, and the Hydrocephalus Association all contributed to this research.