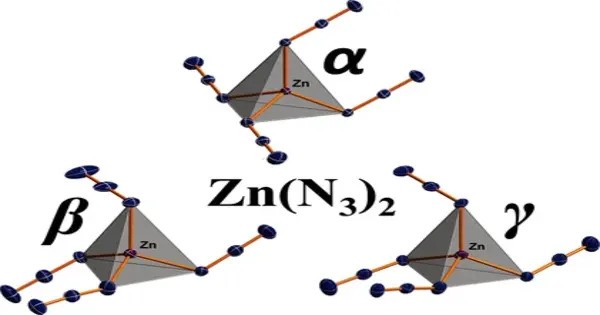
Zinc azide Zn(N3)2 is an inorganic compound composed of zinc cations (Zn2+) and azide anions (N−3). It is an example of a metal azide, where the azide group (N₃⁻) acts as a ligand coordinating to the metal center, in this case, zinc. It typically appears as a white or colorless crystalline solid.
It is a white, explosive solid that can be prepared by the protonolysis of diethylzinc with hydrazoic acid:
Zn(C2H5)2 + 2 HN3 → Zn(N3)2 + 2 C2H6
Properties
- Chemical formula: Zn(N3)2
- Molar mass: 149.4 g/mol
- Appearance: White solid
- Density: 2.559 g/cm3 (α polymorph)
Occurrences
Zinc azide is not naturally occurring in significant amounts but can be synthesized in laboratories. It is typically produced through the reaction of zinc salts with sodium azide or another azide source.
Preparation:
- One common method of preparation involves reacting zinc sulfate (ZnSO₄) or zinc chloride (ZnCl₂) with sodium azide (NaN₃):
ZnCl2+2NaN3→Zn(N3)2+2NaCl
While zinc azide itself does not occur abundantly in nature, azides in general are used in various synthetic organic reactions and materials science.
Uses
Zinc azide and other azide compounds are mainly of interest in the fields of:
- Explosives: Azides are known for their energetic properties, and some are used in detonators, though zinc azide itself is less commonly employed for this purpose.
- Chemical Synthesis: Azides can be useful intermediates in the synthesis of other compounds, such as those used in pharmaceuticals, dyes, and polymers.
Safety Concerns
Azide compounds, including zinc azide, are highly toxic and sensitive to physical shock, heat, and friction. Care must be taken when handling them, and appropriate safety protocols should be followed to prevent accidental detonation or poisoning.