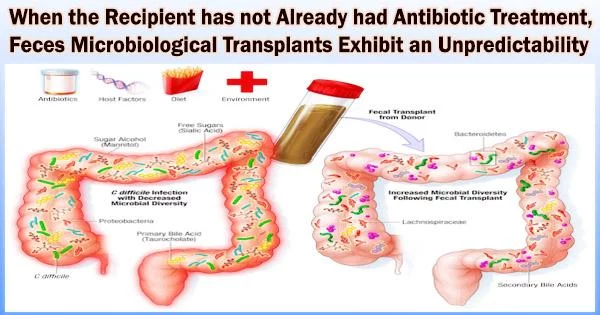
After numerous courses of suppressive antibiotics have completely eliminated the recipient microbial community, a fecal microbial transplant, which involves giving a recipient feces from a donor to alter the recipient’s gut microbial community in the colon, has been a successful last resort treatment for people with recurrent Clostridium difficile infection.
However, fecal microbial transplants have also been used to modify the recipient’s immune to fight cancer or their metabolic to reduce obesity. In these transplants, the recipient is not given suppressive antibiotics to get rid of the microbial population beforehand.
In these situations, the newly transplanted gut community is made up of a mixture of donor and recipient bacteria that must compete as additional microbial strains are added to the already existing community.
Researchers from the University of Alabama at Birmingham have now reported in the journal PLOS One that when there is no preconditioning to reduce the recipient microbe community, it is not predictable for fecal microbial transplants to change the gut microbial community to correspond to that of the donor. In contrast, persistent long-term colonization of donor strains of C. difficile is observed up to two years after transplant in C. difficile fecal microbial
“The practical translation of our analysis suggests the use of pre-fecal microbial transplant treatments to reduce recipient microbial communities to facilitate a donor microbial strain-dominated gut microbial community following fecal microbial transplant,” said UAB researchers Hyunmin Koo, Ph.D., and Casey Morrow, Ph.D.
“In addition, longitudinal sampling of individual fecal microbial transplant patients in combination with strain tracking analysis to monitor the status of the post-fecal microbial transplant microbial community would also be important to assess the stability and, ultimately, the success of the fecal microbial transplant.”
The complex oscillating patterns of the appearance of fecal dominant donor, recipient or unrelated strains following extended times post-fecal microbial transplant provide new insights into the dynamics of the microbial community interactions with the recipients following fecal microbial transplant.
Casey Morrow
Koo and Morrow analyzed metagenomic sequencing published by Davar et al. in Science in 2021, using two established strain tracking methods, the WSS strain-tracking method developed at UAB and the strain-level population genomics tool StrainPhlAn.
WSS can detect whether a donor strain or recipient strain of a particular species of gut microbes is dominant after fecal microbial transplants, and StrainPhlAn provides a phylogenetic tree of donor-related microbes or recipient-related microbes.
Since it had been observed that the composition of the gut microbiota correlates with the efficacy of anti-PD-1 therapy in animal models and cancer patients, the Davar group administered fecal microbial transplants from patients who responded to anti-PD-1 immunotherapy for melanoma to recipient patients who had been resistant to the immunotherapy. Six out of 15 patients benefited.
Koo and Morrow analyzed five fecal microbial transplants by the Davar group where the recipient microflora was sampled multiple times for as long as 535 days post-fecal microbial transplant. This published metagenomic data allowed a time-series strain tracking analysis.
The UAB researchers found that three Alistipes bacterial species and one Parabacteroides species all had patterns, post-fecal microbial transplant, of either dominant donor or dominant recipient strains in the feces.
The dominance of either the donor or recipient fecal strains was evident in Bacteroides uniformis and Bacteroides vulgatus, in contrast, which displayed inter-individual oscillation over time. The fact that some dominating strains of the two Bacteroides species were unrelated to either the donor or the recipient strains added to the complexity.
Also, one Bacteroides vulgatus strain showed a possible genetic recombination event between the donor and recipient strains.
“The complex oscillating patterns of the appearance of fecal dominant donor, recipient or unrelated strains following extended times post-fecal microbial transplant provide new insights into the dynamics of the microbial community interactions with the recipients following fecal microbial transplant,” Morrow said.
“The result from our analysis has implications for the use of fecal microbial transplant to predictably change the biological functions of the gut community in metabolism and host immunity.”
Morrow is professor emeritus of the UAB Department of Cell, Developmental and Integrative Biology, and Koo is bioinformatician in the UAB Department of Genetics. Both departments are in the Marnix E. Heersink School of Medicine.