Most bacteria may form colonies, known as biofilms, that attach to a wide range of surfaces and are difficult to remove. This can cause severe issues, such as in hospitals or the food sector. An international team led by Hebrew University in Jerusalem and the Technical University Dresden studied a model system for biofilms at the synchrotron radiation facilities BESSY II at HZB and the ESRF and discovered what role the structures within the biofilm play in the distribution of nutrients and water.
Bacterial biofilms can grow on practically any surface, including rocks and plants, teeth and mucous membranes, but also contact lenses, medical implants or catheters, dairy industry hoses, and drinking water pipes, where they can pose a major hazard to human health. Some biofilms are also useful in the creation of cheese, where specific forms of biofilms not only produce many tiny holes but also supply delightful flavor.
Tissue with special structures
“Biofilms are a tissue with particular structures, not merely a collection of many bacteria,” argues Prof. Liraz Chai of the Hebrew University in Jerusalem. Bacteria work together to build an extracellular matrix, which is a protective covering of carbohydrates and proteins. This matrix shields the bacteria from disinfectants, UV radiation, and desiccation, making biofilms extremely difficult to remove mechanically or chemically.
Biofilms are tissue with particular structures, not merely a collection of many bacteria. We were surprised because spores usually grow in response to stress, such as dehydration. However, they are related with water routes here, most likely due to metal ion accumulation.
Prof. Liraz Chai
The matrix, however, is not a homogeneous sludge: “It’s a bit like a plant leaf, there are specialized structures, such as water conduits residing in microscopic creases,” adds Chai. But until recently, no one knew what role these structures play or what occurred at the molecular level in a biofilm. Chai applied for measurement time at the synchrotron radiation source BESSY II at HZB with Prof. Yael Politi, TU Dresden, an expert in the characterization of biological materials.
“The advantage of BESSY II is that we can map quite large areas; by combining X-ray diffraction with fluorescence, we can not only analyze the molecular structures across the biofilm very precisely, but we can also track the accumulation of certain metal ions that are transported in the biofilm and learn about some of their biological roles,” Yael Politi explains.
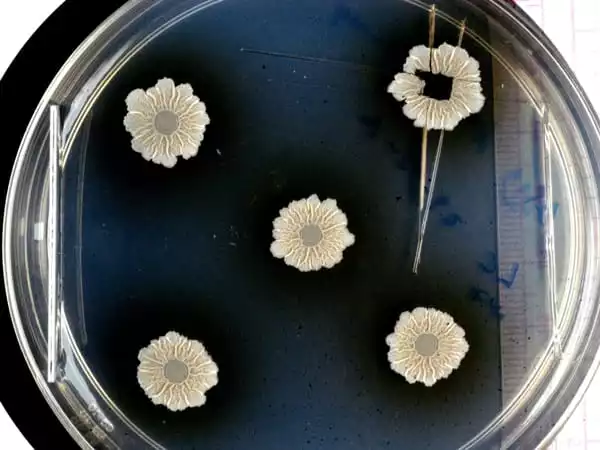
Model system for many biofilms
Biofilms from Bacillus subtilis, a harmless bacteria that grows on plant roots and creates a valuable symbiosis with them: it stores water so that the plant might perhaps extract moisture from the biofilm during drought, and it also protects the roots from infections, were used as samples. In exchange, the biofilm cells feed on root exudates. Nonetheless, Bacillus subtilis bacteria can be used as a model system for a variety of different bacterial biofilms.
They studied a broad area (mm2) of these biofilm samples using the BESSY II MySpot beamline. They were able to spatially resolve the biofilm formations and discriminate between matrix components, bacterial cells, spores, and water. “X-ray fluorescence spectroscopy,” explains Dr. Ivo Zizak, HZB scientist in charge of the MySpot beamline, “is a method that allows us to identify essential metal ions such as calcium, zinc, manganese, and iron,” even when present in tiny amounts. This allowed researchers to establish a link between biofilm morphology and metal ion distribution.
Spore formation at unexpected locations
The analysis demonstrates that calcium ions preferentially accumulate in the matrix, whereas zinc, manganese, and iron ions accumulate along with the wrinkles, where they may promote the development of spores, which are critical for bacterial dispersal.
“We were surprised because spores usually grow in response to stress, such as dehydration. However, they are related with water routes here, most likely due to metal ion accumulation” says Chai.
The findings reveal that the matrix structures not only play a vital function in the delivery of nutrients and water but also actively influence the bacteria’s capacity to behave as a multicellular organism. “This could help us deal with biofilms in general, both good and detrimental,” adds Liraz Chai.