Trinickel boride is a compound of nickel and boron with the chemical formula Ni3B. It is the common name of materials composed chiefly of the elements nickel and boron that are widely used as catalysts in organic chemistry. It is one of the borides of nickel.
The compound was described in 1959 by R. Fruchart, S. Rundquist, and L. H. Anderson, and R. Kiessling. It is a hard solid with a cementite crystal structure. These compounds are useful industrial materials due to their microhardness, high chemical and thermal stability, excellent selectivity and activity for liquid-phase reactions, and their effectiveness as catalysts.
Synthesis
Trinickel boride can be obtained, as grains embedded in a nickel matrix, by heating Brown’s P-1 and P-2 “nickel boride” catalyst to 250 °C. The functionality of Ni-B compounds is dependent on the form—amorphous or crystalline—often determined by the method of synthesis. This catalyst is produced by the reduction of nickel salts with sodium borohydride.
Trinickel boride can be obtained also by compressing nickel and boron powders with explosives. Crystalline forms of Ni-B are determined by sintering temperatures and heating duration applied to a precursor material which may include amorphous Ni-B compositions.
Recently it has been found that Ni3B can be formed (together with other nickel borides) by heating sodium borohydride with powdered nickel metal to 670 °C in a closed vessel so that the released hydrogen creates a pressure of up to 3.4 MPa. The main reactions can be summarized as
2NaBH4 ↔ 2NaH + B2H6
3Ni + 2B2H6 + NaH ↔ Ni3B + 3BH3 + 2H2 + Na
but other reactions occur, yielding other borides.
Solid-state synthesis of boron compounds is usually undertaken at high temperatures due to the high melting point of boron. For example, Ni3B is synthesized at 1000 °C, Ni2B at 1200 °C, and Ni4B3 at 750–900 °C.
Safety
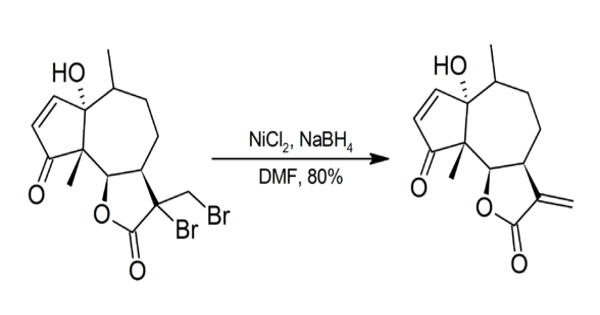
Nickel compounds are possible carcinogens and contact with the skin should be avoided. Particular care should be taken whenever NiCl2/NaBH4 is used in DMF as sodium borohydride may spontaneously ignite in DMF.