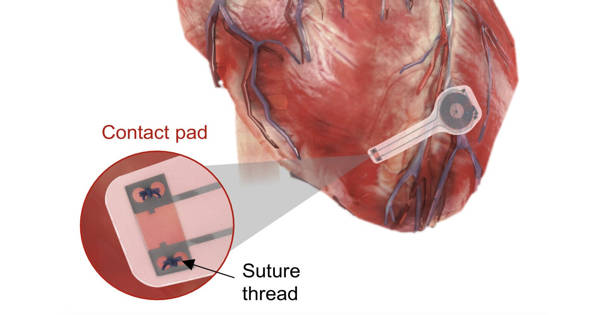
The thin, flexible, lightweight device could be used in patients who require temporary pacing following cardiac surgery or while awaiting a permanent pacemaker. All pacemaker components are biocompatible and will naturally dissolve into the body’s biofluids over the course of five to seven weeks without the need for surgical extraction.
Northwestern and George Washington (GW) University researchers have created the first-ever transient pacemaker, which is a wireless, battery-free, fully implantable pacing device that disappears when it is no longer needed.
The thin, flexible, lightweight device could be used in patients who require temporary pacing following cardiac surgery or while awaiting a permanent pacemaker. All pacemaker components are biocompatible and will naturally dissolve into the body’s biofluids over the course of five to seven weeks without the need for surgical extraction.
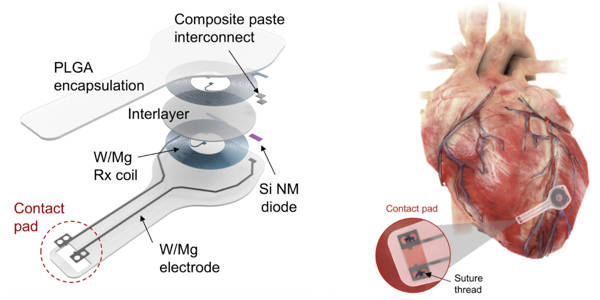
Using near-field communication protocols, the device wirelessly harvests energy from an external, remote antenna – the same technology used in smartphones for electronic payments and RFID tags. This eliminates the need for bulky batteries as well as rigid hardware such as wires (or leads). Not only can leads introduce infections, they also can become enveloped in scar tissue, causing further damage when removed.
The study was published in the journal Nature Biotechnology. The paper demonstrates the device’s efficacy across a series of large and small animal models.
Researchers developed the first-ever transient pacemaker – a wireless, battery-free, fully implantable pacing device that disappears after it’s no longer needed.
“Hardware placed in or near the heart increases the risk of infection and other complications,” said John A. Rogers of Northwestern University, who led the device’s development. “Our wireless, transient pacemakers overcome key disadvantages of traditional temporary devices by eliminating the need for percutaneous leads for surgical extraction procedures, potentially resulting in lower costs and better patient outcomes. This novel device could be the future of temporary pacing technology.”
“Sometimes patients only require pacemakers for a short period of time, such as after open-heart surgery, a heart attack, or a drug overdose,” said Dr. Rishi Arora, a cardiologist at Northwestern Medicine who co-led the study. “We can remove the pacemaker once the patient’s heart has stabilized. The current standard of care entails inserting a wire that remains in place for three to seven days. These have the potential to become infected or to become dislodged.”
“The transient electronics platform opens an entirely new chapter in medicine and biomedical research,” said Igor Efimov, a GW researcher who co-led the study with Rogers and Arora. “The bioresorbable materials at the heart of this technology enable the development of a plethora of diagnostic and therapeutic transient devices for monitoring disease and therapy progression, delivering electrical, pharmacological, cell therapies, gene reprogramming, and more.”
Rogers is the director of the Querrey Simpson Institute for Bioelectronics and the Louis Simpson and Kimberly Querrey Professor of Materials Science and Engineering, Biomedical Engineering, and Neurological Surgery at the McCormick School of Engineering and the Feinberg School of Medicine. Arora is a Feinberg professor of medicine and the co-director of the Center for Arrhythmia Research. Efimov is the Alisann and Terry Collins Professor of Biomedical Engineering at George Washington University.
Ditching restrictive, risky leads
Currently, surgeons must sew temporary pacemaker electrodes onto the heart muscle during surgery to set up temporary pacing after open-heart surgery. These have leads that exit the front of the patient’s chest and connect to an external pacing box, which delivers a current to control the heart’s rhythm.
When the temporary pacemaker is no longer required, the pacemaker electrodes are removed by doctors. Infection, dislodgement, torn or damaged tissues, bleeding, and blood clots are all possible complications of implanted temporary pacemakers.
Surgeons and patients can avoid this potentially dangerous procedure with Northwestern and GW’s transient pacemaker. The fully implantable device is small and light, measuring 250 microns in thickness and weighing less than half a gram. It is soft and flexible, encasing electrodes that softly laminate onto the surface of the heart to deliver an electrical pulse.
“Instead of using wires that can become infected and dislodged,” Arora explained, “we can implant this leadless biocompatible pacemaker.” “The circuitry is directly implanted on the surface of the heart, and we can activate it remotely.” This new type of pacemaker ‘dissolves’ or degrades on its own over a period of weeks, eliminating the need for physical removal of the pacemaker electrodes. This could be a significant victory for post-operative patients.
“With further advancements, such bioresorbable pacemakers may eventually be implantable through a vein in the leg or arm,” he added. “In this case, it may also be possible to provide temporary pacing to patients who have had a heart attack or who are undergoing catheter-based procedures such as transcatheter aortic valve replacement.”
Prioritizing patient comfort
Dr. Duc Thinh Pham, a Northwestern Medicine cardiac surgeon who was not involved in the study, believes a transient pacemaker would undoubtedly make his patients more comfortable. Patients with current pacemakers frequently experience discomfort for several days after the leads are inserted. Then, in order to keep the leads from dislodging, they must restrict their movements and activities.
“This transient pacemaker is brilliant,” Pham, who has performed over 2,000 cardiac surgeries in his career, said. “In addition to addressing the primary issue of post-cardiac surgery patients who require temporary pacing due to blockages or arrhythmias, the device addresses the secondary issue of patient comfort, freedom of movement, and rehabilitation. If this device is successful, it will significantly improve a patient’s postoperative course.”
Disappearing act
This is the second instance of bioresorbable electronic medicine from the Rogers lab, which has been researching transient electronics for over a decade. Rogers and colleagues demonstrated the world’s first bioresorbable electronic device in 2018 – a biodegradable implant that accelerates nerve regeneration. The bioresorbable devices developed by the team are completely safe, similar to absorbable stitches. The devices completely disappear after fully degrading due to the body’s natural biological processes.
“There is clearly a need for better temporary cardiac pacemakers,” said Dr. Bradley Knight, the Chester C. and Deborah M. Cooley Distinguished Professor of Cardiology at Feinberg and coauthor of the study.
“When I first learned about the bioresorbable nerve stimulator, I contacted Professor Rogers to see if this technology could be used to pace the heart. As a proof of concept, he had already begun working with Dr. Efimov to develop a small version of a bioresorbable pacemaker. We then collaborated with both teams to create a larger version of a bioresorbable, leadless cardiac pacemaker that could be used on humans. It’s an excellent example of what Northwestern can achieve by combining engineering and medical expertise.”
Depending on the patient, a temporary pacemaker may be required for a few days to several weeks. Rogers’ team can control the number of days the device remains functional before dissolving by varying the composition and thickness of the materials in it.
“We build these devices out of various types of safe, bioresorbable materials and in optimized architectures to ensure stable operation over a time period that is slightly longer than clinically necessary,” Rogers explained. “We can customize the devices to address a wide range of relevant lifetimes. In general, transient technologies could one day provide therapy or treatment for a wide range of medical conditions, essentially serving as an engineering form of medicine.”