Isomers are molecules that have the same molecular formula but have a different arrangement of the atoms in space. A structural isomer, or constitutional isomer, is a type of isomer in which molecules with the same molecular formula have different bonding patterns and atomic organization, as opposed to stereoisomers, in which molecular bonds are always in the same order and only spatial arrangement differs. Structural isomers have the same molecular formula but a different bonding arrangement among the atoms. They are molecules with the same molecular formula, but their atoms have different arrangements or bonds. There are multiple synonyms for structural isomers. They have both cationic and anionic parts as complexes with a different distribution of ligands and/or metal ions are known as coordination isomers. They differ from stereoisomers, which share the same chemical formulas and the same order of atoms, but have different three-dimensional configurations. Examples
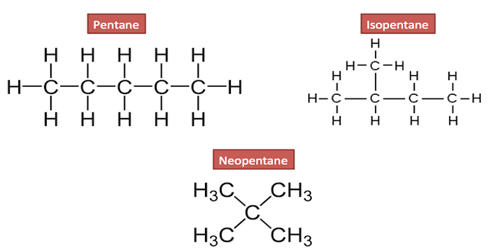
- Butane and isobutane (C4H10) are structural isomers of each other.
- Pentan-1-ol, pentan-2-ol, and pentan-3-ol are structural isomers that exhibit position isomerism.
- Cyclohexane and hex-1-ene are examples of functional group structural isomers.
Isomers are compounds with different physical and chemical properties but the same molecular formula. Structural isomers are molecules with the same molecular formula, but their atoms have different arrangements or bonds. They are molecules that have the same molecular formula but with the atoms connected in a different order. They are isomers that have the same component atoms but they are arranged differently from each other. Three categories of structural isomers are skeletal, positional, and functional isomers. They are compounds with different carbon skeletons, different functional groups, and different functional group locations. Positional isomers are also called regioisomers. For example – Butane and isobutane have the same number of carbon (C) atoms and hydrogen (H) atoms, so their molecular formulas are the same.
There are three types of structural isomers.
- Chain isomers – In chain isomers, the carbon atoms are connected in different orders. It is a type of structural isomerism where the isomers have same molecular formula but they differ in the order in which the carbon atoms are bonded to each other.
- Position isomers – In position isomers, the carbon skeleton remains unchanged, but functional groups are moved around. For example, there are two structural isomers that occur in n-butanol with the molecular formula C4H9OH.
- Functional Group Isomers – In functional group isomers, the atoms are arranged to make different functional groups. For example, there are two functional group isomers found with the molecular formula C2H6O.