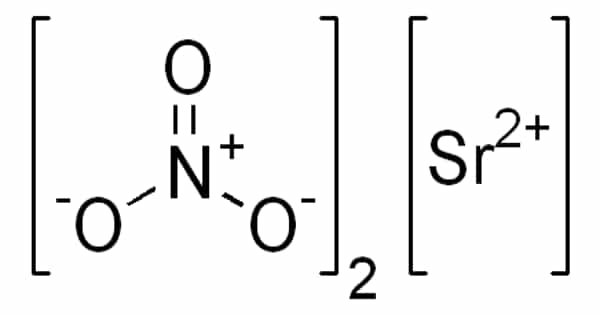Strontium nitrate is an inorganic compound with the formula Sr(NO3)2 that is made up of the elements strontium, nitrogen, and oxygen. It is an inorganic compound composed of two elements, namely nitrogen and strontium. In pyrotechnics, this colorless solid is used as a red colorant and oxidizer.
If large quantities are involved in a fire or the combustible material is finely divided, it may explode. It may explode if exposed to heat or fire for an extended period of time. In fires, toxic nitrogen oxides are produced. It is used in pyrotechnics, medicine, and the production of other chemicals.
Properties
Strontium nitrate appears as a white crystalline solid. Noncombustible but accelerates burning of combustible materials. It is a crystalline solid white in color. It is a noncombustible compound but enhances the burning of combustible compounds.
- Melting point: 570 °C (lit.)
- Boiling point: 645 °C
- Density: 2.99.
- Solubility: 660g/l
- Form: Solid
- Specific Gravity: 2.99
- Color: White
- Odor: Odorless

Preparation
Strontium nitrate is typically generated by the reaction of nitric acid on strontium carbonate.
2 HNO3 + SrCO3 → Sr(NO3)2 + H2O + CO2
If there is an excess of nitrate de strontium in the fire, it can explode. It can also explode if exposed to heat for an extended period of time. When burned, it emits toxic nitrogen oxides.
Uses
Strontium nitrate, like many other strontium salts, is used to produce a rich red flame in fireworks and road flares. This salt’s oxidizing properties are useful in such applications.
Strontium nitrate can help to eliminate and reduce skin irritations. Strontium nitrate, when combined with glycolic acid, significantly reduces the sensation of skin irritation compared to glycolic acid alone.
Biochemistry
Sr2+ ions, as a divalent ion with an ionic radius similar to Ca2+, have the ability to traverse calcium-selective ion channels and trigger neurotransmitter release from nerve endings. As a result, it is used in electrophysiology experiments.