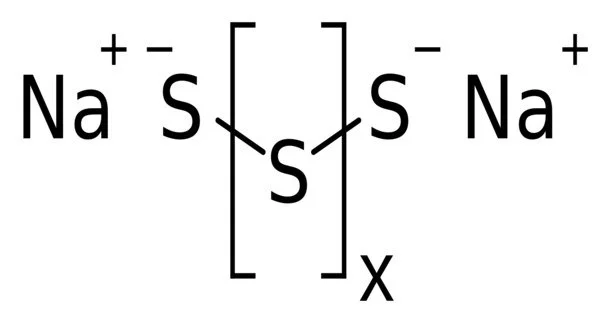Sodium polysulfide refers to salts with the formula Na2Sx, where x ranges from 2 to 5. The polysulfide anions Sx2− comprise disulfide (S22−), trisulfide (S32−), tetrasulfide (S42−), and pentasulfide (S52−). The chain lengths might be longer in theory, but not in practise. The salts are dark red solids that, when dissolved in water, form highly alkaline and corrosive solutions. These salts oxidise in air and hydrolyze to produce hydrogen sulphide.
Sodium polysulfides are used in a variety of sectors, including the manufacture of chemicals, dyes, and flotation agents for mineral processing. They’re also employed in wastewater treatment and as additives in some electrochemical processes.
Properties
- Color and Physical State: It can be yellow or orange solids, depending on the sulfur content. In their pure form, they are generally crystalline substances.
- Odor: These have a characteristic odor, often described as a rotten egg smell, which is common to many sulfur compounds.
- Solubility: These are soluble in water to varying degrees, depending on the length of the polysulfide chain (number of sulfur atoms). Shorter chain polysulfides are more soluble than longer chain ones.
- Reducing Agent: They act as reducing agents, meaning they can donate electrons during chemical reactions, leading to the reduction of other substances.
- Stability: These are sensitive to air and moisture, and their stability depends on the length of the polysulfide chain. Longer chain polysulfides are generally more stable.
Structure
The polysulfide anions form chains with S—S bond distances around 2 Å in length. The chains adopt skewed conformations. In the solid state, these salts are dense solids with strong association of the sodium cations with the anionic termini of the chains.
Preparation
The reaction of sodium sulphide (Na2S) with elemental sulphur (S) yields sodium polysulfide. The number of sulphur atoms in the molecule (x) determines the specific properties and applications of sodium polysulfide.
Production and occurrence
Sulphur can be converted into sodium polysulfide by dissolving it in a sodium sulphide solution. Alternatively, they are generated at high temperatures by the redox reaction of aqueous sodium hydroxide with sulphur. Finally, they are formed via the reduction of elemental sulphur with sodium, which is frequently carried out in anhydrous ammonia.
These salts are used to make polysulfide polymers, as a chemical fungicide, as a blackening agent on copper jewellery, as a component in a polysulfide bromide battery, as a toner in a photochemical solution, and to remove hair from hides in the tanning business.
Reactions
As exploited in the sodium-sulfur battery, the polysulfides absorb and release reducing equivalents by breaking and making S-S bonds, respectively. An idealized reaction for sodium tetrasulfide is shown:
Na2S4 + 2 Na ⇌ 2 Na2S2
Alkylation gives organic polysulfides according to the following idealized equation:
Na2S4 + 2 RX → 2 NaX + R2S4
Alkylation with an organic dihalide gives polymers called thiokols.
Protonation of these salts gives hydrogen sulfide and elemental sulfur, as illustrated by the reaction of sodium pentasulfide:
Na2S5 + 2 HCl → H2S + 4 S + 2 NaCl
Application
One of sodium polysulfide’s most important applications is in the paper and pulp industry, where it is used as a pulping agent to break down lignin and aid in the separation of cellulose fibres during the paper-making process. It’s also employed in wastewater treatment, as a reducing agent in several chemical reactions, and to make sulphur dyes. It is critical to handle sodium polysulfide with caution since it includes sulphide ions, which are poisonous and create hydrogen sulphide gas when exposed to acids. Proper safety precautions and instructions should be observed when handling and using it.