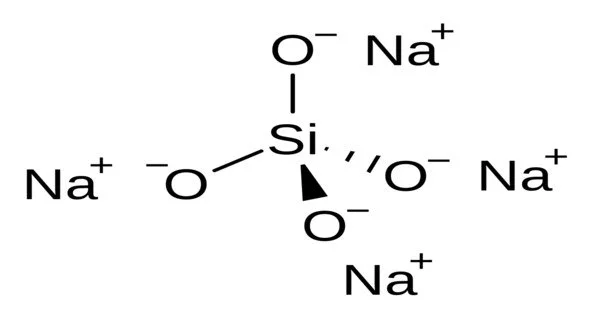
The chemical compound sodium orthosilicate has the molecular formula Na4SiO4. It’s a sodium silicate, more specifically an orthosilicate, which is a salt of the unstable orthosilicic acid H4SiO4. It is a silicate, which are minerals or compounds that contain silicon and oxygen. It is one of many silicate compounds, each with a unique structure and set of properties.
Properties
- Chemical composition: The compound consists of four sodium ions (Na+) and one orthosilicate ion (SiO4) in its formula, represented as Na4SiO4.
- Solubility: It is soluble in water, and its solubility increases with temperature. It readily dissolves in water to form a colorless to slightly yellowish solution.
- Silicate chemistry: Silicates, in general, are essential components of many minerals, rocks, and materials found in the Earth’s crust. They are significant contributors to the geology and chemistry of the planet.
Natural occurrence
Sodium orthosilicate has not been found in nature. However, the mineral chesnokovite, chemically the related salt disodium dihydrogen orthosilicate [Na+]2[SiO2(OH)2−2] · 8H2O, was recently identified in the Kola Peninsula.
Uses
In the water-flooding of oil fields for enhanced oil extraction, sodium orthosilicate has been considered as an interfacial tension-reducing additive. It was discovered in laboratory settings to be more effective than sodium hydroxide for some types of oil.
As an anticorrosion treatment for iron and steel surfaces, sodium orthosilicate has been found to stabilize ferrate films. It has numerous applications in various industries. It is commonly used in detergent production because it can act as a builder, assisting in the removal of mineral deposits and improving cleaning efficiency. It is also used in ceramics and as a raw material in the synthesis of other chemicals.