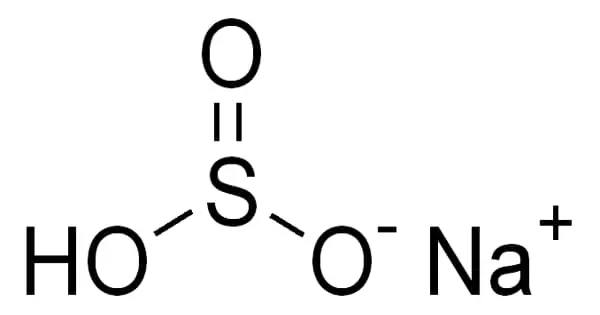
Sodium bisulfite is a chemical compound with the approximate chemical formula NaHSO3. It appears as white crystals or crystalline powder. Sodium bisulfite is not a real compound, but rather a mixture of salts that dissolve in water to form solutions containing sodium and bisulfite ions. It is a common inorganic salt that is also known as sodium hydrogen sulfite. It’s a white solid with a sulfur dioxide odor.
It is used directly as a bacterial inhibitor in the distilled beverage industries. Sodium bisulfite is also found in the potato, shrimp, and cherry processing industries.
Properties
Sodium bisulfite is a white solid with a mild sulfurous odor. Its density is 1.48 g/mL and its melting point is 150 °C. It is highly soluble in water. Aqueous solutions of NaHSO4 are highly acidic in nature. Chemically it is an acidic salt, rather than a usual neutral salt.
- Molecular Weight: 104.06
- Appearance: White powder or cvrystals
- Melting Point: Decomposes
- Boiling Point: N/A
- Density: 1.48 g/cm3
- Solubility in H2O: N/A
- Exact Mass: 103.954409

Synthesis
Sodium bisulfite solutions can be prepared by treating a solution of a suitable base, such as sodium hydroxide or sodium bicarbonate with sulfur dioxide.
SO2 + NaOH → NaHSO3
SO2 + NaHCO3 → NaHSO3 + CO2
Attempts to crystallize the product yield sodium disulfite, Na2S2O5.
Sodium bisulfite is formed during the Wellman-Lord process.
Preparation
The reaction of an acid and a base that results in the formation of salt and water. The reaction of sulfuric acid (H2SO4) and sodium hydroxide (NaOH) can produce NaHSO4, but these elements must be reacted in a specific ratio: the acid must be abundant in comparison to the base.
Industrially, sodium bisulfite is produced by passing sulfur dioxide gas through aqueous solutions of bases such as sodium hydroxide or sodium bicarbonate.
SO2 + NaOH → NaHSO3
Reactivity
Sodium bisulfite is a water-dissolved form of sodium meta-bisulfite. As a reducing agent, it can supply sulfur dioxide to applications where a liquid source would be preferable.
Sodium bisulfite is a common industrial reducing agent, as it readily reacts with dissolved oxygen: 2 NaHSO3 + O2 → 2 NaHSO4
It is typically used to prevent oxidative corrosion in large piping systems. Maintaining anaerobic conditions within a reactor is beneficial in biochemical engineering applications. It is a critical component in the production of dye intermediates. There are also significant applications in the processing of wool and the antichlor treatment of many textile products. Sodium bisulfite is an important component of water treatment.
Uses
- Sodium bisulfite is used as a food additive and a food preservative.
- It is used in purification and decolorization processes during production of various chemicals.
- It has been used externally for parasitic skin diseases and as gastrointestinal antiseptic.
- It has applications in wastewater treatment, in drinking water treatment, in prevention of corrosion, wine making, and DNA sequencing.
- It is also used as a flame retardant, anti-scaling agent and a bleaching agent.
Health effects/safety hazards
Sodium bisulfite powder or concentrated sodium bisulfite solutions can be extremely irritating to the skin, eyes, throat, and mucous membranes. If swallowed, it can cause severe diarrhea or even death. It reacts violently with acids, releasing a pungent sulfur dioxide gas that is extremely irritating to the eyes and mucous membranes.