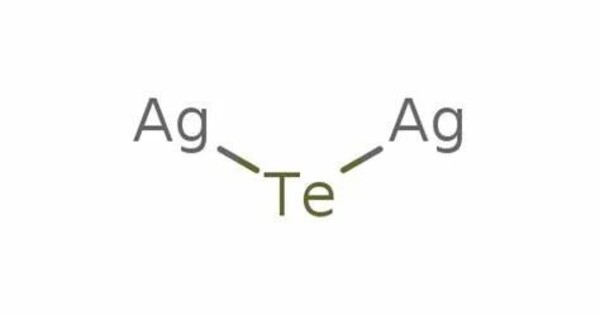
Silver telluride (Ag2Te) is a chemical compound, a telluride of silver, also known as disilver telluride or silver(I) telluride. It’s a semiconductor with interesting properties, particularly in thermoelectric applications, where it can convert temperature differences into electrical voltage. It forms a monoclinic crystal. In a wider sense, silver telluride can be used to denote AgTe (silver(II) telluride, a metastable compound) or Ag5Te3. This makes it useful for power generation and refrigeration technologies.
Properties
Silver(I) telluride occurs naturally as the mineral hessite, whereas silver(II) telluride is known as empressite. It is a semiconductor which can be doped both n-type and p-type. Stoichiometric Ag2Te has n-type conductivity. On heating silver is lost from the material.
- Chemical formula: Ag2Te
- Molar mass: 341.3364 g/mol
- Appearance: grey-black crystals
- Density: 8.318 g/cm3
- Melting point: 955 °C (1,751 °F; 1,228 K)
Occurrences
Silver telluride occurs naturally in mineral deposits, often in association with other silver and tellurium minerals. It can be found in the following minerals: Argentite (Ag₂S) and Tellurite (TeO₂).
- Geological Settings: Typically found in epithermal veins and hydrothermal deposits. It is often formed in areas with volcanic activity or in association with other metal deposits.
- Commercial Extraction: Silver telluride is mined as a byproduct in silver mining operations, particularly in regions rich in tellurium-bearing ores. It is of interest for its potential use in thermoelectric devices due to its semiconducting properties.
Applications
- Thermoelectric Materials: Used in devices that convert heat to electricity and vice versa.
- Optoelectronics: Explored for use in infrared detectors and other electronic components.