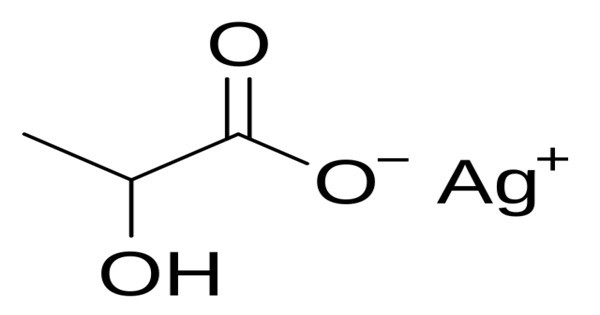
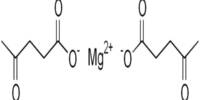
Silver lactate is an organic chemical compound, a salt of silver and lactic acid with the formula CH3CH(OH)COOAg. It is part of a group of silver salts, with silver being known for its antimicrobial properties. The lactate component is derived from lactic acid, which is a naturally occurring organic acid found in various biological systems.
Properties
Silver lactate forms light gray crystals. It is soluble in water, slightly soluble in ethanol. It forms a crystalline hydrate of composition CH3CH(OH)COOAg•H2O. It is a reagent for the precipitation of uric acid.
- Chemical formula: CH3CH(OH)COOAg
- Molar mass: 196.93 g/mol
- Appearance: Gray to purple powder or flakes
- Melting point: 120–122 °C (248–252 °F; 393–395 K)
- Boiling point: 227.6 °C (441.7 °F; 500.8 K)
- Solubility in water: Soluble
Chemical properties
The compound reacts with triphenylphosphine gold chloride in a mixed solvent of benzene and dichloromethane to obtain colorless triphenylphosphine gold lactate. The compound reacts with a tetraphosphine ligand, dppbpda, to obtain a coordination polymer [(dppbpda)Ag4(CH3CH(OH)COO)4]n.
Synthesis
Silver lactate can be made by the reaction of silver carbonate with lactic acid. Silver lactate is soluble in water, which makes it easier to incorporate into solutions or formulations. Compared to other forms of silver, silver lactate is considered to be less toxic, which is why it’s used in certain medical treatments.
Uses
- Medical and Antiseptic Applications: Due to its antimicrobial nature, silver lactate is used in some topical ointments and wound care products, helping to prevent infection.
- Cosmetics: It is sometimes incorporated into personal care products, like lotions and creams, for its antibacterial properties.
- Food Preservation: Though less common, silver lactate has been explored as a preservative due to its ability to inhibit microbial growth.
- Pharmaceuticals: It has been studied in the formulation of silver-based antibiotics and other drugs.
Safety Considerations
While silver lactate is generally regarded as safe for topical use, excessive silver exposure (through misuse or prolonged contact) can lead to a condition known as argyria, where silver builds up in the body and causes a bluish-gray discoloration of the skin.