A team of researchers at Los Alamos National Laboratory has developed a way to use photochemistry to separate plutonium and uranium—work that could make it easier to store nuclear waste. In their paper published in the journal Chemical Communications, the group describes their purification process.
In order to use plutonium in weapons or in electricity-generating plants, it must first be purified. One common purification process involves the separation of plutonium and uranium. This is typically done through the use of redox agents applied during actinide separation. Afterward, chemical agents are applied to bind the actinides to allow for separation.
The photochemistry process resulted in light-induced redox reactions without the need for other agents, so less toxic waste was produced. In research work, the team showed that the use of energy-efficient LEDs results in a more efficient process.
Unfortunately, this process creates a large amount of hazardous waste, typically in liquid form, which is stored in barrels. Storage of such waste has become politically divisive. For that reason, chemists have been searching for a cleaner way to separate plutonium and uranium that does not produce hazardous waste products. In this new effort, the team in New Mexico developed a photochemistry-based method that partly meets that specification.
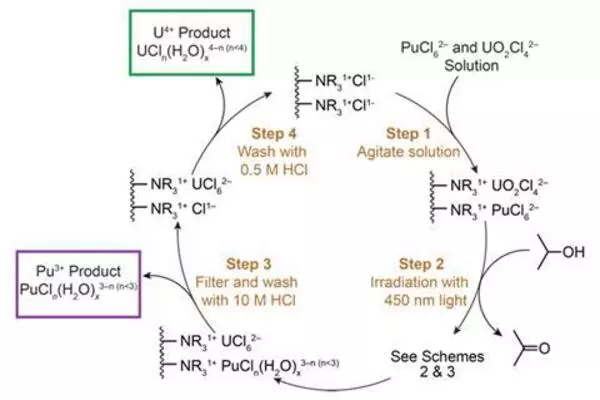
Prior research has shown that the application of photochemistry to uranium processing is possible under certain circumstances. More specifically, researchers have found that both visible and ultraviolet light can be used to tune oxidation states. Other experiments have shown that similar processes could be used to separate actinides.
In their work, the researchers used UV-vis light to induce plutonium4+ and uranium6+ into becoming plutonium3+ and uranium4+. This allowed the use of more traditional separation methods to complete the purification process.
The photochemistry process resulted in light-induced redox reactions without the need for other agents, so less toxic waste was produced. In their work, the team showed that the use of energy-efficient LEDs results in a more efficient process. The researchers suggest their process is a candidate for piggybacking onto work by the larger catalysis community studying the possibilities of light-induced reactions.