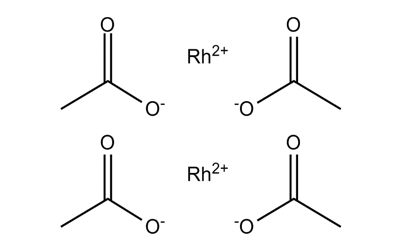
Rhodium (II) acetate
Rhodium is a member of the platinum group of metals. It has a higher melting point than platinum, but a lower density. Rhodium (II) acetate is the chemical compound with the formula Rh2(AcO)4, where AcO− is the acetate ion (CH3CO−2). This dark green powder is slightly soluble in polar solvents, including water. It is used as a catalyst for the cyclopropanation of alkenes. Acetates are excellent precursors for the production of ultra-high purity compounds, catalysts, and nanoscale materials. Rhodium(II) Acetate Dimer is a moderate water-soluble crystalline Rhodium source that decomposes to Rhodium oxide on heating. It is generally immediately available in most volumes.
Rhodium (II) acetate is a dark green powder that is slightly soluble in polar solvents, including water. It is used as a catalyst for the cyclopropanation of alkenes.
Preparation
Rhodium is found in ores mixed with other metals such as palladium, silver, platinum, and gold. Rhodium (II) acetate is usually prepared by the heating of hydrated rhodium (III) chloride in acetic acid (CH3COOH): Rhodium(II) acetate dimer undergoes ligand exchange, the replacement of the acetate group by other carboxylates and related groups.
Rh2(OAc)4 + 4 HO2CR → Rh2(O2CR)4 + 4 HOAc
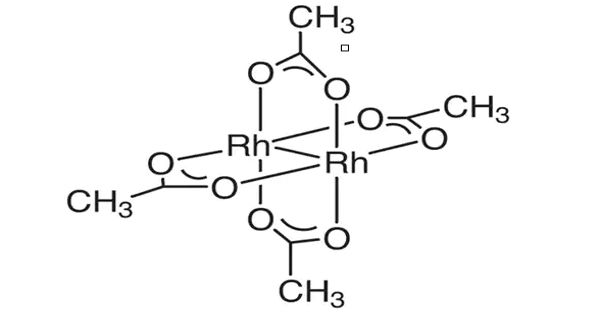
Structure and properties
It is a moderately water-soluble crystalline Rhodium source that decomposes to Rhodium oxide on heating. It is generally immediately available in most volumes. The structure of rhodium(II) acetate features a pair of rhodium atoms, each with octahedral molecular geometry, defined by four acetate oxygen atoms, water, and an Rh–Rh bond of length 2.39 Å. The water adduct is exchangeable, and a variety of other Lewis bases bind to the axial positions. Copper(II) acetate and chromium (II) acetate adopt similar structures.
Chemical properties
Rhodium Acetate Solutions are moderate to highly concentrated liquid solutions of Rhodium Acetate. They are an excellent source of Rhodium Acetate for applications requiring solubilized materials. The application of dirhodium tetraacetate to organic synthesis was pioneered by Teyssie and co-workers. An extensive range of reactions including insertion into bonds and the cyclopropanation of alkenes and aromatic systems. It selectively binds ribonucleosides (vs. deoxynucleosides) by binding selectively to ribonucleosides at their 2′ and 3′ –OH groups.
Rhodium is primarily used as the catalyst in the three-way catalytic converters of automobiles it is also highly valued in jewelry. The name Rhodium originates from the Greek word ‘Rhodon,’ which means rose. Acetates are excellent precursors for the production of ultra-high purity compounds and certain catalyst and nanoscale (nanoparticles and nanopowders) materials. Acetates are also proving useful in the field of solar energy technologies.