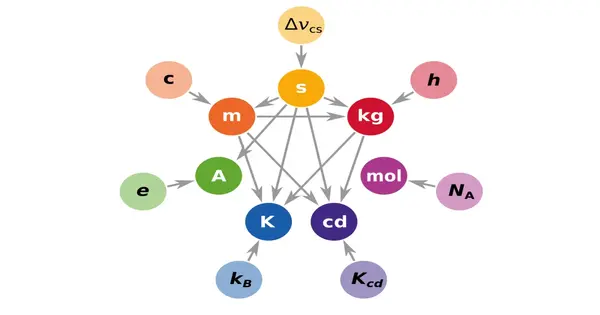
SI unit names are always written in lowercase when written. The symbols of units named after people, on the other hand, are capitalized (e.g., ampere and A). Because these are not abbreviations, no periods are required. In addition, except for the degree symbol, a space should always be included between a number and the SI unit. Italics are rarely used with SI units.
Rules and conventions for writing SI units and their symbols –
(1) The units named after scientists are not written with a capital initial letter.
For example : newton, henry, watt
(2) The symbols of the units named after scientist should be written by a capital letter.
For example : N for newton, H for henry, W for watt
(3) Small letters are used as symbols for units not derived from a proper name.
For example : m for metre, kg for kilogram
(4) No full stop or other punctuation marks should be used within or at the end of symbols.
For example : 50 m and not as 50 m.
(5) The symbols of the units do not take plural form.
For example : 10 kg not as 10 kgs
(6) When temperature is expressed in kelvin, the degree sign is omitted.
For example : 273 K not as 273o K (If expressed in Celsius scale, degree sign is to be included. For example 100o C and not 100 C)
(7) Use of solidus is recommended only for indicating a division of one letter unit symbol by another unit symbol. Not more than one solidus is used.
For example : m s−1 or m / s, J / K mol or J K–1 mol–1 but not J / K / mol.
(8) Some space is always to be left between the number and the symbol of the unit and also between the symbols for compound units such as force, momentum, etc.
For example, it is not correct to write 2.3m. The correct representation is 2.3 m; kg m s–2 and not as kgms-2.
(9) Only accepted symbols should be used.
For example : ampere is represented as A and not as amp. or am ; second is represented as s and not as sec.
(10) Numerical value of any physical quantity should be expressed in scientific notation.
For an example, density of mercury is 1.36 × 104 kgm−3 and not as 13600 kg m−3.