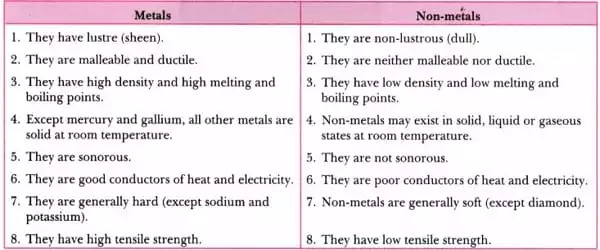
Identification of metals and nonmetals can be difficult if you are unfamiliar with their properties. A metal is a solid substance that is usually hard, lustrous, and opaque. A non-metal, on the other hand, is a solid or gaseous material that lacks metallic properties.
Metals have the ability to be drawn into wires due to ductility, but nonmetals do not. Sonorous refers to the ability of metals to produce a deep or ringing sound. Non-metals, on the other hand, are not sonorous. Metals have exceptionally high melting and boiling points. Non-metals, on the other hand, are boiled and melted at relatively low temperatures.
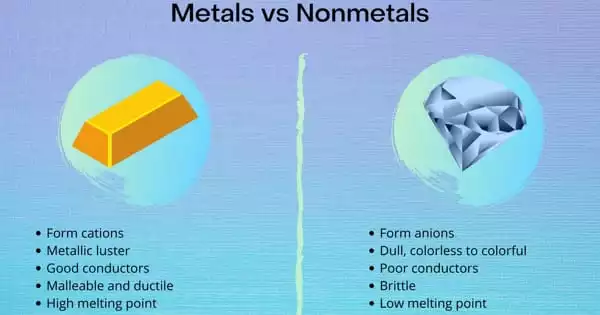
Difference between Metals and Non-metals –
METALS
- Metals are naturally occurring elements that are hard, shiny, opaque, and dense. Metals are natural elements that are hard, shiny, opaque, and dense.
- Metals are natural elements that are solid, lustrous, opaque, and have a higher density. Metals have extremely high melting and boiling points. They are excellent conductors of heat and electricity. The atoms in metals are arranged in a crystal structure. They function as reducing agents by losing valence electrons and forming cations. Silver, aluminum, gold, lead, nickel, copper, titanium, magnesium, iron, cobalt, zinc, and other metals are examples.
- Metals are electropositive in nature because they lose electrons easily, making them reducing agents. Metals are usually solid at room temperature, with the exception of mercury and gallium, which are liquids.
- Metals are hard and are commonly used in the manufacture of machinery, water boilers, agricultural equipment, automobiles, industrial equipment, utensils, and airplanes, among other things. Metals combine with dilute acid to form salt and hydrogen gas.
NON-METALS
- Chemical substances that are soft, non-shiny, transparent, and brittle are referred to as non-metals. Metals are natural elements that are hard, shiny, opaque, and dense. Non-metals are chemical substances that are soft, non-shiny, transparent, and brittle.
- Non-metals, as the name implies, are natural elements that do not have metallic properties. Except for bromine, which is the only nonmetal that exists in liquid form, these are usually present in solid or gaseous form. They are soft, non-lustrous (except for iodine), and excellent heat and electricity insulators. For example, nitrogen, oxygen, hydrogen, argon, xenon, chlorine, and so on.
- Nonmetals are electronegative because they gain electrons and thus act as oxidizers. Except for bromine, which is the only nonmetal that exists in liquid form, these can be found in solid or gaseous form.
- Atoms in nonmetals are arranged in a non-crystalline or amorphous structure. Because they gain or share valence electrons to form anions, nonmetals have a high ionisation energy and electronegativity. Because they are typically soft, they are used in the production of fertilizer, water purification, crackers, and other products.
Metals are smooth and shiny, whereas nonmetals are usually dull. Metals are generally hard substances in terms of hardness, but this varies from substance to substance. Except for diamond, which is the hardest substance on the planet, nonmetals are soft.