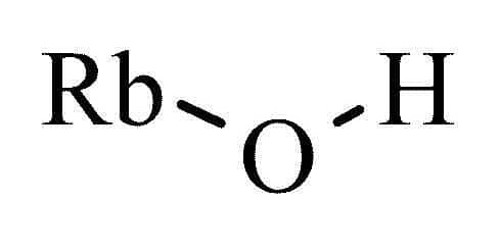Rubidium hydroxide is a chemical compound. It is composed of rubidium and hydroxide ions. Its chemical formula is [RbOH]. It is commercially available from a certain number of chemical suppliers in the form of a 50% or 99% aqueous solution. However, it can be obtained by synthesis from rubidium oxide.
Rubidium hydroxide can be synthesized by carefully adding rubidium oxide into the water:
Rb2O + H2O → 2 RbOH
Boiling a solution of rubidium carbonate with calcium hydroxide will also yield rubidium hydroxide.
It is a strong base and alkali that is formed by one rubidium ion and one hydroxide ion. It can eat through the glass. It is made by dissolving rubidium oxide in water. It causes immediate burns to the skin. It gets very hot when it is dissolved in water. It is a white solid.
Properties
Rubidium hydroxide appears as a grayish-white solid. It is an alkali metal hydroxide and a rubidium molecular entity. It is a white hygroscopic solid, which is very soluble in water.
- Chemical formula: [RbOH]
- Molar mass: 102.475 g/mol
- Appearance: grayish-white solid, hygroscopic
- Density: 1.74 g/mL at 25 °C
- Melting point: 301 °C (574 °F; 574 K)
- Boiling point: 1,390 °C (2,530 °F; 1,660 K)
- Solubility in water: 100 g/100 mL (15 °C)
- Solubility: soluble in ethanol
Uses
Rubidium hydroxide is rarely used in industrial processes because potassium hydroxide and sodium hydroxide can perform nearly all the industrial functions of rubidium hydroxide in a less violent and hence safer way.
It is used in electric storage batteries. It is used in scientific research. It is often used sparingly to prevent waste of the expensive element rubidium. For example, it is used to give fireworks a violet color in place of pure rubidium.
Hazard prevention
Rubidium hydroxide is a corrosive compound that causes immediate burns to the skin on contact. It is very irritating to skin and eyes. Denser than water. Ultimate care must be carried out when handling this chemical.