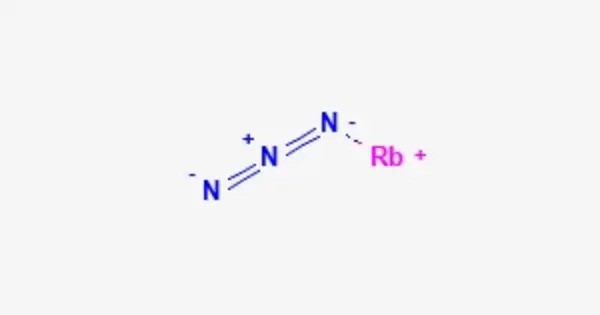
Rubidium azide is an inorganic compound with the formula RbN3. It is the rubidium salt of the hydrazoic acid HN3. It appears as a white crystalline solid, and like other alkali metal azides, it is highly reactive, especially when exposed to heat or shock. It can decompose rapidly, releasing nitrogen gas (N₂), which is why it’s considered hazardous. Like most azides, it is explosive.
Rubidium azide may be used in specialty applications, like in research, but it is most notably a precursor to the synthesis of other compounds, especially rubidium salts.
Properties
Rubidium azide is typically a white, crystalline solid. It is highly soluble in water, which is a characteristic of many alkali metal azides. It is sensitive to heat and can decompose when exposed to high temperatures, releasing nitrogen gas (N₂) and possibly rubidium oxide (Rb₂O).
- Chemical formula: RbN3
- Molar mass: 127.49 g⋅mol−1
- Appearance: Colorless needles
- Density: 2.79 g⋅cm−3
- Melting point: 317–321 °C (603–610 °F; 590–594 K)
- Solubility in water: 107.1 g/100 g (16 °C), 114.1 g/100 g (17 °C)
- Solubility: 0.182 g/100 g (16 °C, ethanol)
Preparation
Rubidium azide can be created by the reaction between rubidium sulfate and barium azide which results in formation of easily separated insoluble barium sulfate:
Rb2SO4 + Ba(N3)2 → 2 RbN3 + BaSO4
In at least one study, rubidium azide was produced by the reaction between butyl nitrite, hydrazine monohydrate, and rubidium hydroxide in the presence of ethanol:
C4H9ONO + N2H4·H2O + RbOH → RbN3 + C4H9OH + 3 H2O
This formula is typically used to synthesize potassium azide from caustic potash.
Reactions
As with all azides, it will decompose and release nitrogen gas when heated or severely shocked:
2 RbN3 → 2 Rb + 3 N2
Discharge rubidium azide in nitrogen gas will produce rubidium nitride.
Occurrences
Rubidium azide does not occur naturally in large quantities. As an inorganic compound, it is generally synthesized in laboratories or industrial settings. It is not commonly found in minerals or natural deposits but can be created by reacting rubidium salts (like rubidium hydroxide or rubidium carbonate) with sodium azide (NaN₃) or other azide salts.
Safety
Due to the instability of azides, rubidium azide must be handled with caution. Azides can be explosive under the right conditions, and exposure to high heat, shock, or friction may lead to dangerous reactions.
Uses
Rubidium azide has been investigated for possible use in alkali vapor cells, which are components of atomic clocks, atomic magnetometers and atomic gyroscopes. Azides are desirable starting materials because they decompose into rubidium metal and nitrogen gas when exposed to UV light.
- Explosive/propellant: Rubidium azide, like other alkali metal azides, has been explored for use in explosive materials or as a propellant.
- Chemical research: It is used in research to explore the reactivity and properties of azides, particularly in synthetic chemistry and materials science.
- Biochemistry: Some azides, including rubidium azide, have been used in biological studies, though they must be handled with care due to their toxicity and instability.