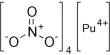DNA repair is an important biological process that helps cells maintain the integrity of their genetic material. There are various mechanisms for repairing various types of DNA damage, such as chemical modifications, breaks, and cross-links. A new study adds to an emerging, radically different picture of how bacterial cells repair faulty sections of their DNA on a continuous basis.
The report, which was published online in the journal Cell, describes the molecular mechanism behind a DNA repair pathway that prevents the incorrect inclusion of a specific type of molecular building block, ribonucleotides, into genetic codes. Such errors are common in the code-copying process of bacteria and other organisms. Because ribonucleotide misincorporation can cause harmful DNA code changes (mutations) and DNA breaks, all organisms have evolved a DNA repair pathway called ribonucleotide excision repair (RER) that quickly corrects such errors.
Last year a team led by Evgeny Nudler, Ph.D., the Julie Wilson Anderson Professor in the Department of Biochemistry and Molecular Pharmacology at NYU Langone Health, published two analyses of DNA repair in living E. coli cells. They found that most of the repair of certain types of DNA damage (bulky lesions), such as those caused by UV irradiation, can occur because damaged code sections have first been identified by a protein machine called RNA polymerase. RNA polymerase motors down the DNA chain, reading the code of DNA “letters” as it transcribes instructions into RNA molecules, which then direct protein building.
Our results continue to inspire a rethinking of certain basic principles in the DNA repair field. Moving forward, our team plans to investigate whether RNA polymerase scans DNA for all kinds of problems and triggers repair genome-wide, not only in bacteria, but in human cells as well.
Evgeny Nudler
Nudler and colleagues discovered that during the transcription process, RNA polymerase detects DNA lesions and acts as a platform for the assembly of a DNA repair machine known as the nucleotide excision repair (NER) complex. NER then extracts the faulty DNA and replaces it with an accurate copy. Little, if any, NER occurs in living bacteria without the action of RNA polymerase.
The new Cell study provides the first evidence that RER, like the NER pathway, is tightly coupled to transcription. The researchers discovered evidence that RNaseHII, the key enzyme involved in RER, collaborates with RNA polymerase as it scans for misincorporated ribonucleotides in the DNA chains of living bacterial cells.
“Our results continue to inspire a rethinking of certain basic principles in the DNA repair field,” says Nudler, also an investigator with the Howard Hughes Medical Institute. “Moving forward, our team plans to investigate whether RNA polymerase scans DNA for all kinds of problems and triggers repair genome-wide, not only in bacteria, but in human cells as well.”
Cutting Edge Techniques
Ribonucleotides (RNA building blocks) and deoxyribonucleotides (DNA components) are chemically related. According to the study authors, when bacterial cells copy and build DNA chains, they frequently incorporate ribonucleotides instead of deoxyribonucleotides because they differ by only one oxygen atom. Every time DNA polymerase III copies a cell’s genetic material, it is known to make approximately 2,000 of these errors. The RER pathway removes the majority of misplaced ribonucleotides to maintain genome integrity, but a key question had been how RNaseHII finds relatively rare ribonucleotide lesions in an “ocean” of intact cellular DNA codes so quickly.
As they did in their 2022 studies, the researchers used quantitative mass spectrometry and in vivo protein-protein crosslinking to map the distances between chemically linked proteins, and so determined the key surfaces of RNaseHII and RNA polymerase as they interact in living bacterial cells. In this way they determined that most RNaseHII molecules couple with RNA polymerase.
They also used cryogenic electron microscopy (CryoEM) to capture high-resolution structures of RNaseHII bound to RNA polymerase in order to reveal the protein-protein interactions that define the RER complex. Furthermore, RER was jeopardized by structure-guided genetic experiments that weakened the RNA polymerase/RNaseHII interaction.
“This work supports a model in which RNaseHII scans DNA for misplaced ribonucleotides while riding on RNA polymerase,” says first study author Zhitai Hao, a post-doctoral scholar in Nudler’s lab. “This research is critical for our basic understanding of the DNA repair process and has broad clinical implications.”