Researchers at the University of Cologne have found a protein complex that hinders the repair of genome damage in human cells, mice, and the nematode Caenorhabditis elegans. Also, they used a pharmacological drug to successfully block this complex for the first time.
“When we suppress the so-called DREAM complex in body cells, various repair mechanisms kick in, making these cells extremely resilient towards all kinds of DNA damage,” said Professor Dr. Björn Schumacher, Director of the Institute for Genome Stability in Aging and Disease at the University of Cologne’s CECAD Cluster of Excellence in Aging Research.
Our DNA needs to be carefully safeguarded because it includes all of our genetic information. Yet, external factors or our regular metabolism constantly threaten to harm it. Hence, DNA repair is crucial for maintaining the integrity of our genome and maintaining cellular function.
“Our findings for the first time allow us to improve DNA repair in body cells and to target the causes of aging and cancer development,” Schumacher added. Still, more research is needed until these results can be translated into new therapies for human patients. The study ‘The DREAM complex functions as conserved master regulator of somatic DNA repair capacities’ has appeared in Nature Structural & Molecular Biology.
Caenorhabditis elegans is a small, free-living, transparent nematode (roundworm) that is widely used as a model organism in biology, genetics, and developmental biology. It is a multicellular organism with a simple body plan that consists of approximately 1,000 somatic cells and a nervous system consisting of 302 neurons.
DNA damage leads to aging and disease
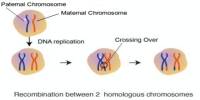
Our genetic material is passed on from generation to generation. That is why it is particularly well protected in our germ cells. There, relatively few mutations in the genetic code are passed on to progeny thanks to very accurate DNA repair systems.
We were very pleased to see the same effect as we did in C. elegans. The human cells were much more resilient towards DNA damage after treatment.
Arturo Bujarrabal
Our human genome has been passed down to us by our ancestors for two hundred thousand years thanks to DNA repair. It has consistently made sure that the genetic data is kept safe. Our bodily cells likewise constantly repair DNA, but only for as long as the person is alive.
Sometimes, children are born with damaged DNA repair systems, which causes them to age more quickly and develop age-related disorders including arteriosclerosis and neurodegeneration even as young children. In some cases, they also have an extremely increased risk of cancer. These are all consequences of DNA damage not being properly repaired.
The DREAM complex prevents repairs
Schumacher and his team investigated why body cells do not have the same healing mechanisms as germ cells. They discovered that the DREAM protein complex, which attaches to the DNA’s construction plans providing instructions for the repair mechanisms, limits the number of DNA repair mechanisms in body cells through tests with the nematode C. elegans. This prevents them from being produced in large quantities.
C. elegans has a short life cycle of around three days, which makes it an ideal organism for genetic studies. It is also easy to maintain in the laboratory and can be grown on bacterial plates. Because of its transparency, its development and behavior can be observed easily under a microscope.
Germ cells, however, do not have the DREAM complex. Hence, they naturally produce large quantities of DNA repair mechanisms.
Mammals also have a DREAM complex
The researchers demonstrated that the DREAM complex operates in human cells in the same manner through additional laboratory (cell culture) studies using human cells. Also, they were able to use a pharmacological drug to override the DREAM complex.
“We were very pleased to see the same effect as we did in C. elegans. The human cells were much more resilient towards DNA damage after treatment,” said Arturo Bujarrabal, a postdoc in Schumacher’s team and lead author of the study.
Treatment with the DREAM complex inhibitor also showed amazing effects in mice: The DNA in the retina of mice could be repaired and the function of the eye preserved.
The experiment was conducted on mice, which, like certain human patients, experience early aging and exhibit typical retinal degeneration.
Due to the extremely high radiation levels in space, genome damage also plays a significant part in manned spaceflight. A longer stay in space without improved DNA repair is hardly imaginable.
Schumacher says, “Therapies that target and improve this newly discovered master regulator of DNA repair could reduce the risk of cancer because genes remain intact.”
Also, because cells can only perform their role with an intact genome, the risk of age-related disorders would be decreased.