Urea is a carbon, nitrogen, oxygen, and hydrogen-containing organic compound. It is a waste product of protein metabolism that is commonly found in biological systems. While urea is not thought to be a key component in the origin of life, similar compounds such as cyanamide have been proposed to have played a role in the early stages of life’s development. Under the conditions that existed when our planet was first formed, urea reacts extremely quickly. This new discovery adds to our understanding of how life on Earth may have begun.
Researchers from ETH Zurich and the University of Geneva have developed a new method for observing chemical reactions in liquids with extremely high temporal resolution. This means they can study how molecules change in femtoseconds, or quadrillionths of a second. The method is based on previous work by the same group of researchers, led by Hans Jakob Wörner, Professor of Physical Chemistry at ETH Zurich. This research produced similar results for reactions that occur in gas environments.
To extend their X-ray spectroscopy observations to liquids, the researchers needed to create an apparatus capable of producing a liquid jet with a diameter of less than one micrometre in a vacuum. This was essential because if the jet were any wider, it would absorb some of the X-rays used to measure it.
A whole host of important chemical reactions take place in liquids – not just all biochemical processes in the human body, but also a great many chemical syntheses relevant to industry. That is why it is critical that we have now expanded the scope of X-ray spectroscopy at high temporal resolution to include liquid reactions.
Hans Jakob Wörner
Molecular pioneer in biochemistry
The researchers were able to gain insights into the processes that led to the emergence of life on Earth by using the new method. Many scientists believe that urea played an important role in this. It is one of the most basic molecules because it contains both carbon and nitrogen. Furthermore, it is highly likely that urea was present even when the Earth was very young, as suggested by a famous experiment conducted in the 1950s in which American scientist Stanley Miller created a mixture of the gases thought to have made up the planet’s primordial atmosphere and exposed it to thunderstorm conditions. This resulted in the formation of a series of molecules, one of which was urea.
According to current theories, urea could have become enriched in warm puddles on the then-lifeless Earth, known as primordial soup. The concentration of urea in this soup increased as the water evaporated. It’s possible that this concentrated urea produced malonic acid through multiple synthesis steps after being exposed to ionising radiation like cosmic rays. As a result, the building blocks of RNA and DNA may have been formed.
Why this exact reaction took place
The researchers from ETH Zurich and the University of Geneva used their new method to investigate the first step in this long series of chemical reactions to determine how a concentrated urea solution behaves when exposed to ionising radiation.
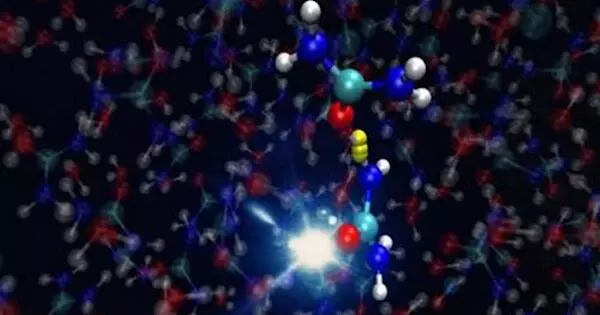
It’s critical to understand that the urea molecules in a concentrated urea solution form pairs, known as dimers. Ionising radiation causes a hydrogen atom within each of these dimers to move from one urea molecule to the other, as the researchers have now demonstrated. One urea molecule is converted into a protonated urea molecule, while the other is converted into a urea radical. The latter is highly chemically reactive – so reactive, in fact, that it’s very likely to react with other molecules, thereby also forming malonic acid.
The researchers also managed to show that this transfer of a hydrogen atom happens extremely quickly, taking only around 150 femtoseconds, or 150 quadrillionths of a second. “That’s so fast that this reaction preempts all other reactions that might theoretically also take place,” Wörner says. “This explains why concentrated urea solutions produce urea radicals rather than hosting other reactions that would produce other molecules.”
Reactions in liquids are highly relevant
Wörner and his colleagues plan to investigate the next steps in the formation of malonic acid in the future. They hope that this will aid in their understanding of the origins of life on Earth.
Their new method can also be used to investigate the precise sequence of chemical reactions in liquids. “A whole host of important chemical reactions take place in liquids — not just all biochemical processes in the human body, but also a great many chemical syntheses relevant to industry,” Wörner says. “That is why it is critical that we have now expanded the scope of X-ray spectroscopy at high temporal resolution to include liquid reactions.”