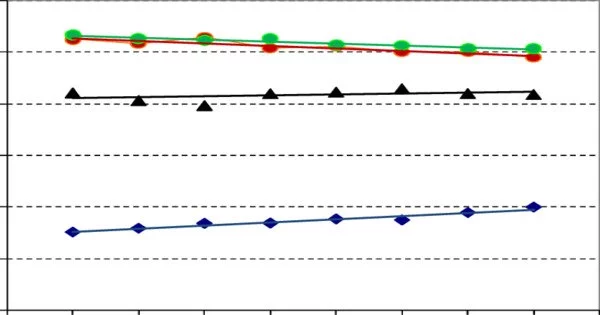
A protocrystalline phase is a unique phase that occurs during crystal formation and eventually transforms into a microcrystalline form. It usually refers to a state or structure that is still in the process of crystallization. Typically, the phrase refers to silicon films used in optical applications such as solar cells. The process through which atoms, ions, or molecules arrange themselves into a highly structured and recurring three-dimensional structure known as a crystal lattice is known as crystallization.
In the early stages of crystallization, the structure may not yet be fully developed or may contain defects.
- Amorphous Materials: In the context of materials science, some substances can exist in an amorphous state, meaning they lack a well-defined crystal structure. When these materials start to develop a crystalline structure, they may be described as “protocrystalline.”
- Geology: In geology, minerals can undergo processes of crystallization, and the term “protocrystalline” might be used to describe minerals that are in the early stages of forming crystals.
- Polymers: In polymer science, some polymers can exhibit a partially ordered structure. When these polymers begin to develop a more ordered and crystalline arrangement of polymer chains, they may be referred to as “protocrystalline polymers.”
The term “protocrystalline” refers to a transitional condition between a disordered or amorphous state and a fully crystalline form. The prefix “proto-” denotes “early or primitive,” hence the crystalline structure is in its early or nascent stages. The degree to which a material is protocrystalline varies and may be affected by factors such as temperature, pressure, and the pace of cooling or solidification.
Applications
Because of its low cost and ease of manufacture, amorphous silicon (a-Si) is a common solar cell material. Because of its disordered structure (Urbach tail), its absorption extends to energies below the band gap, resulting in a broad spectrum response; nonetheless, its solar cell efficiency is rather low.
Because of its more organized crystalline structure, protocrystalline Si (pc-Si:H) has a relatively low absorption near the band gap. Thus, protocrystalline and amorphous silicon can be coupled in a tandem solar cell, where the top thin layer of a-Si:H absorbs short-wavelength light while the underlying protocrystalline silicon layer absorbs longer wavelengths.