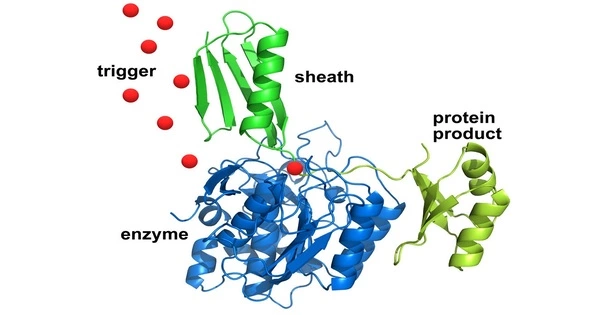
Protein purification is a set of procedures designed to separate one or more proteins from a complicated mixture, typically cells, organs, or entire organisms. It is an important biochemical procedure that involves isolating a specific protein of interest from a complex mixture of proteins. This procedure is required for a variety of applications, including biochemical analysis, structural investigations, and medicinal development.
Protein purification is essential for determining the function, structure, and relationships of a protein of interest. It usually entails a series of procedures that use the protein’s particular features, such as size, charge, solubility, and binding affinity, to separate it from the other components in the mixture.
Here are some commonly used methods and techniques for protein purification:
- Cell Disruption: This step involves breaking open the cells to release the proteins. Various methods, such as mechanical disruption, sonication, or enzymatic lysis, can be used depending on the type of cells and the protein of interest.
- Centrifugation: Based on size and density differences, differential centrifugation can be used to remove cell debris, organelles, and other insoluble material from a protein mixture.
- Fractionation: Techniques such as salting out, in which proteins are precipitated at precise salt concentrations, and organic solvent precipitation can aid in the separation of proteins based on their solubility.
- Chromatography: This is one of the most extensively used protein purification procedures. To exploit differences in features like as charge, size, hydrophobicity, or affinity to specific ligands, various forms of chromatography such as affinity chromatography, ion exchange chromatography, size exclusion chromatography, and hydrophobic interaction chromatography can be used.
- Electrophoresis: Proteins can be separated using techniques such as sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and isoelectric focusing (IEF) depending on their molecular weight and isoelectric point, respectively.
The purification procedure may separate the mixture’s protein and non-protein components, and then separate the required protein from all other proteins. To investigate a protein of interest, it should ideally be segregated from other cell components so that contaminants do not interfere with the examination of the protein’s structure and function.
The most time-consuming element of protein purification is often the separation of one protein from all others. Separation procedures often take advantage of differences in protein size, physicochemical qualities, binding affinity, and biological activity. Protein isolate refers to the pure outcome.
The type of purification chosen is determined by a number of criteria, including the protein’s characteristics, the size of purification required, and the downstream use. To achieve high purity and yield of the desired protein, it is typical to use a mix of these approaches in a purification strategy.