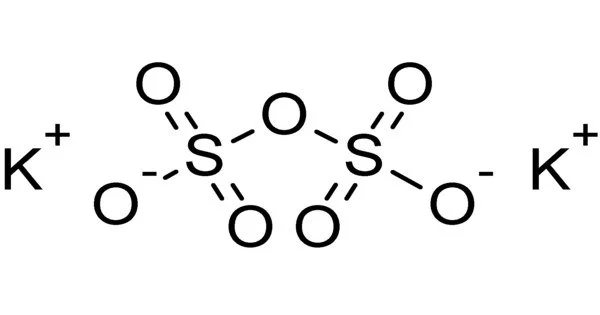
Potassium pyrosulfate has the chemical formula K2S2O7 and is an inorganic compound. It is a white crystalline solid that is extremely water soluble. It is an oxidising agent that, when heated, can release oxygen. It is commonly used as a strong oxidising agent in analytical chemistry and in a variety of laboratory applications. It can be used to oxidise organic compounds as well as to create other chemicals.
Properties
Typically, potassium pyrosulfate exists as a white crystalline solid. It can take the form of colourless to slightly yellow crystals or a white powder. It is only slightly soluble in water. As the temperature drops, so does the solubility of potassium pyrosulfate. It dissolves faster in hot water than cold water. Under normal conditions, it is a stable compound.
- Chemical formula: K2O7S2
- Molar mass: 254.31 g·mol−1
- Density: 2.28 g/cm3
- Melting point: 325 °C (617 °F; 598 K)
- Solubility in water: 25.4 g/100 mL (20 °C)
Production
Potassium pyrosulfate is obtained by the thermal decomposition of other salts, most directly from potassium bisulfate: 2 KHSO4 → K2S2O7 + H2O
Temperatures above 600°C further decompose potassium pyrosulfate to potassium sulfate and sulfur trioxide however: K2S2O7 → K2SO4 + SO3
Other salts, such as potassium trisulfate, can also decompose into potassium pyrosulfate.
Chemical structure
Potassium pyrosulfate contains the pyrosulfate anion which has a dichromate-like structure. The geometry can be visualized as a tetrahedron with two corners sharing the SO4 anion’s configuration and a centrally bridged oxygen atom. A semi-structural formula for the pyrosulfate anion is O3SOSO32−. The oxidation state of sulfur in this compound is +6.
Uses
Potassium pyrosulfate is used in analytical chemistry; samples are fused with potassium pyrosulfate (or a mixture of potassium pyrosulfate and potassium fluoride) prior to quantitative analysis to ensure complete dissolution.
Potassium pyrosulfate is used in a variety of industrial processes. It is used to make dyes, as a catalyst in chemical reactions, and as an oxidising agent in some organic synthesis reactions. It is also useful in the production of other potassium salts. In the industrial production of sulphur trioxide, the compound is also present in a catalyst along with vanadium(V) oxide.
Safety
When handling potassium pyrosulfate, it is important to follow proper safety precautions. It can cause irritation to the skin, eyes, and respiratory system, so it is advisable to wear protective equipment such as gloves, goggles, and a lab coat. Additionally, it should be stored in a cool, dry place away from incompatible substances.