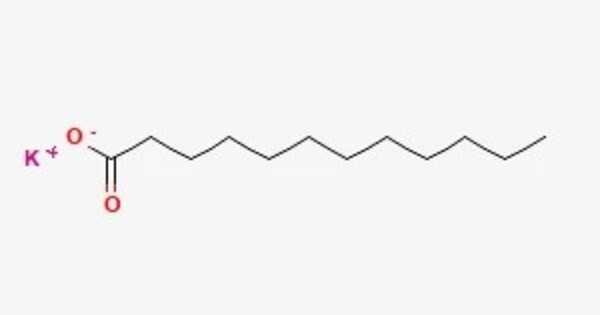
Potassium laurate is a metal-organic compound with the chemical formula C12H23KO2. It is a salt derived from lauric acid and potassium. The compound is classified as a metallic soap, i.e. a metal derivative of a fatty acid (lauric acid). Lauric acid is a type of fatty acid found in coconut oil and palm kernel oil, and potassium laurate is often used in various applications due to its properties as a surfactant and emulsifier.
Potassium laurate is used as a surfactant and emulsifier in various cosmetic formulations such as soaps, shampoos, and lotions. It helps to stabilize emulsions and improve the texture of products. It is used in some cleaning agents and detergents due to its ability to help in the formation of stable foam and its cleansing properties.
Synthesis
Potassium laurate can be prepared via a reaction of lauric acid and potassium hydroxide.
Properties
It is typically a white or off-white powder or solid. It is soluble in water due to the presence of the potassium ion, which increases its solubility compared to sodium laurate. It is soluble in water. It is soluble in ethyl benzene. It forms powder or light-tan paste. It is mildly alkaline in aqueous solutions.
- Chemical formula: C12H23KO2
- Molar mass: 238.41
- Appearance: Powder or light-tan paste
- Melting point: 43.8 °C (110.8 °F; 316.9 K)
- Boiling point: 296.1 °C (565.0 °F; 569.2 K)
- Solubility in water: Soluble
Uses
The compound is used in the cosmetics industry as an emulsifier and surfactant. In cosmetics and personal care products, potassium laurate helps to create stable emulsions, improve the texture, and enhance the foaming properties of products like shampoos, soaps, and lotions. It can also be found in some industrial applications, such as in cleaning products and as a component in certain formulations. Also used as a fungicide, insecticide, and bactericide.